Question: Consider the elementary reversible gas phase reaction. 2 A B + C Where k = 3 . 7 6 4 1 0 - 4 L
Consider the elementary reversible gas phase reaction.
Where If the reaction is to be carried out in
a packed bed reactor at what mass of catalyst is necessary to achieve
conversion? The feed flows at and is comprised of and
balance inert. The inlet pressure is atm and pressure drop across the reactor can be
modeled with the Ergun equation using
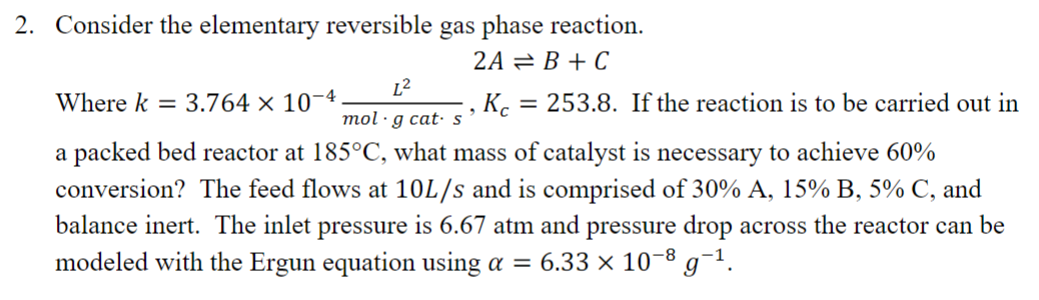
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


