Question: Consider the equimolar binary mixture of n-butane and n-hexane. For the following two sets of conditions, find out the mole fraction of the system
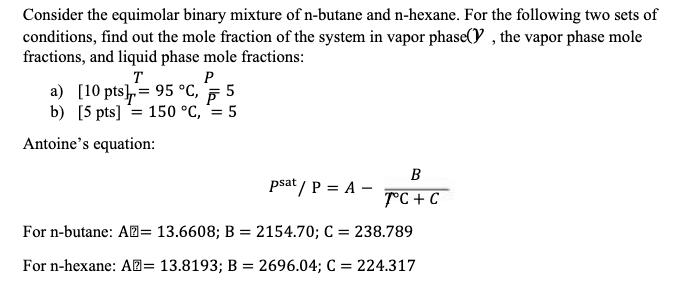
Consider the equimolar binary mixture of n-butane and n-hexane. For the following two sets of conditions, find out the mole fraction of the system in vapor phase(), the vapor phase mole fractions, and liquid phase mole fractions: T a) [10 pts]= 95 C, = b) [5 pts] 150 C, Antoine's equation: P 5 = 5 B psat/P = A- 7C+C For n-butane: A= 13.6608; B = 2154.70; C = 238.789 For n-hexane: A= 13.8193; B = 2696.04; C = 224.317
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


