Question: Consider the following generic reaction. aA+bBcC+dD A plot of log( rate ) versus log[A] when [B] is constant yields the following linear equation for its
![) versus log[A] when [B] is constant yields the following linear equation](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f82c15ad31e_24566f82c1547e86.jpg)
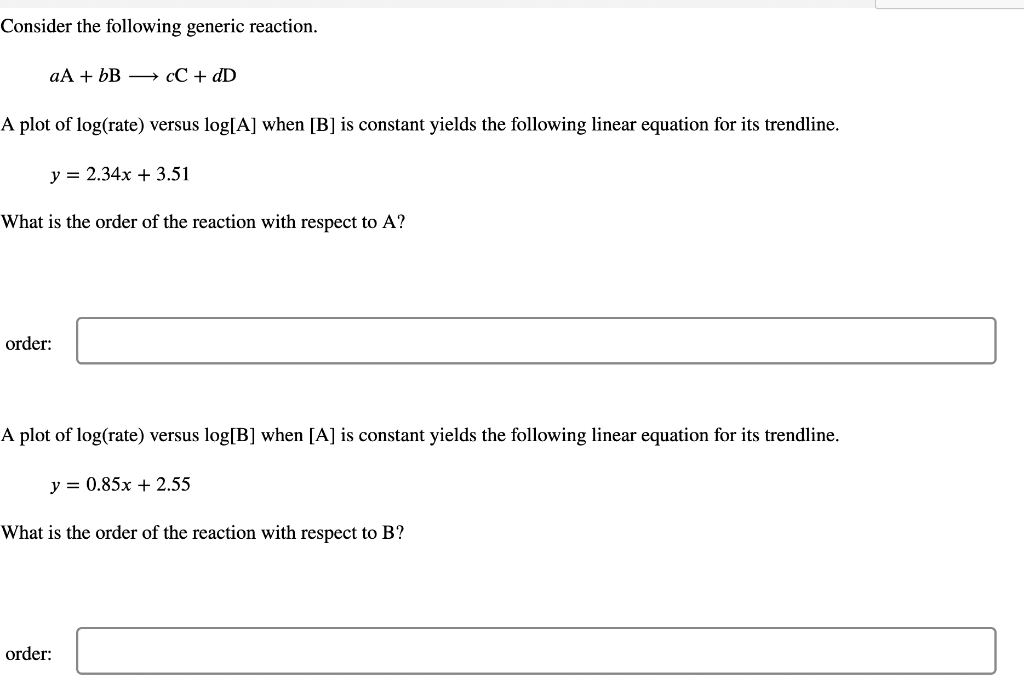
Consider the following generic reaction. aA+bBcC+dD A plot of log( rate ) versus log[A] when [B] is constant yields the following linear equation for its trendline. y=2.34x+3.51 What is the order of the reaction with respect to A ? order: A plot of log( rate ) versus log[B] when [A] is constant yields the following linear equation for its trendline. y=0.85x+2.55 What is the order of the reaction with respect to B ? order What is the overall order of the reaction? overall order: Identify the rate law for this reaction. rate=k[A]2[B]rate=k[A][B]2rate=k[A][B]rate=k[A]2[B]2
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


