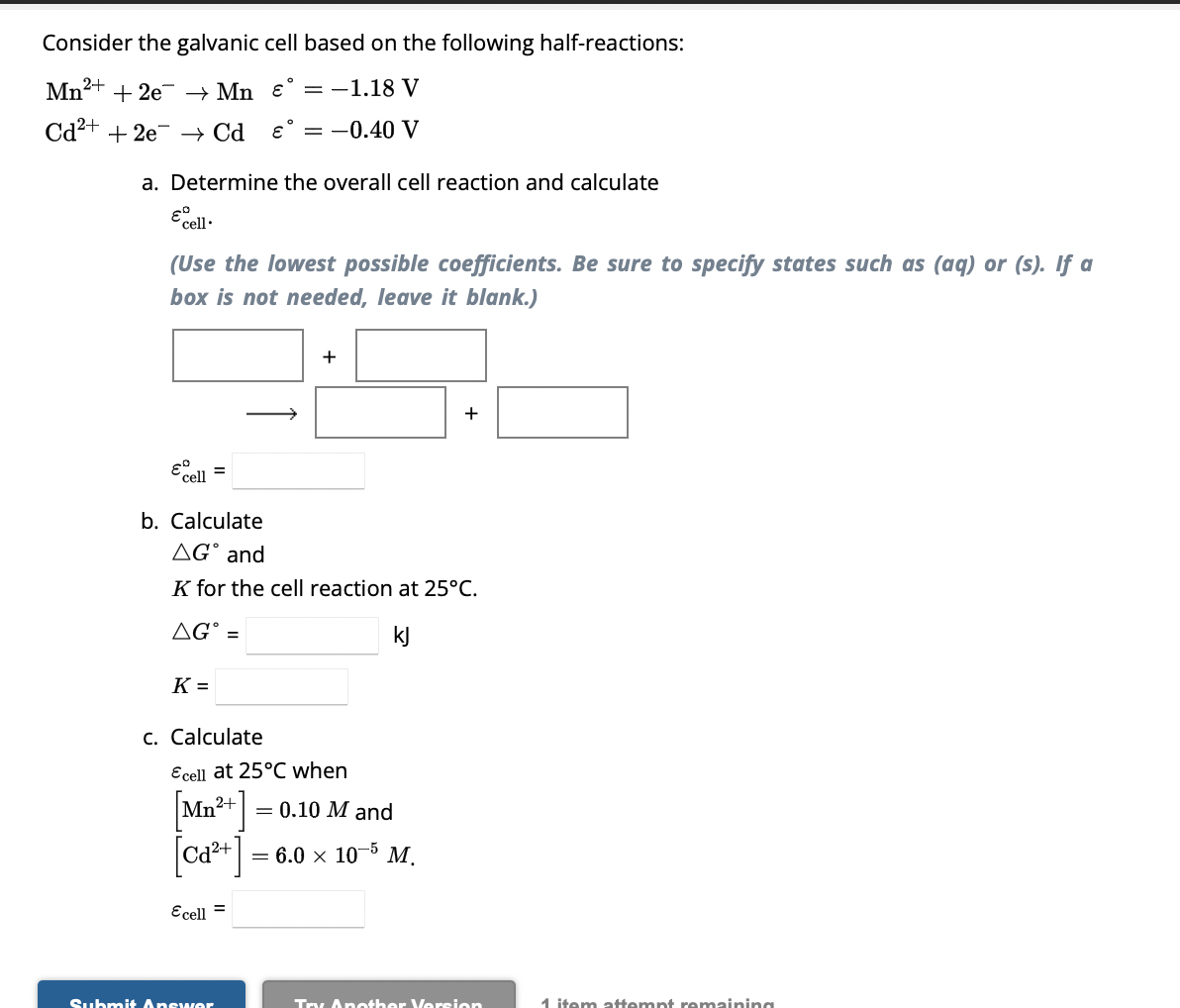
Question: Consider the galvanic cell based on the following half - reactions: M n 2 + + 2 e - M n , = - 1
Consider the galvanic cell based on the following halfreactions:
a Determine the overall cell reaction and calculate
Use the lowest possible coefficients. Be sure to specify states such as aq or s If a
box is not needed, leave it blank.
b Calculate
and
for the cell reaction at
kJ
c Calculate
at when
and
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


