Question: Consider the H2 covalently bonded molecule, with one electron being shared by two positively charged nuclei. Assume the individual wavefunctions w and Up are the
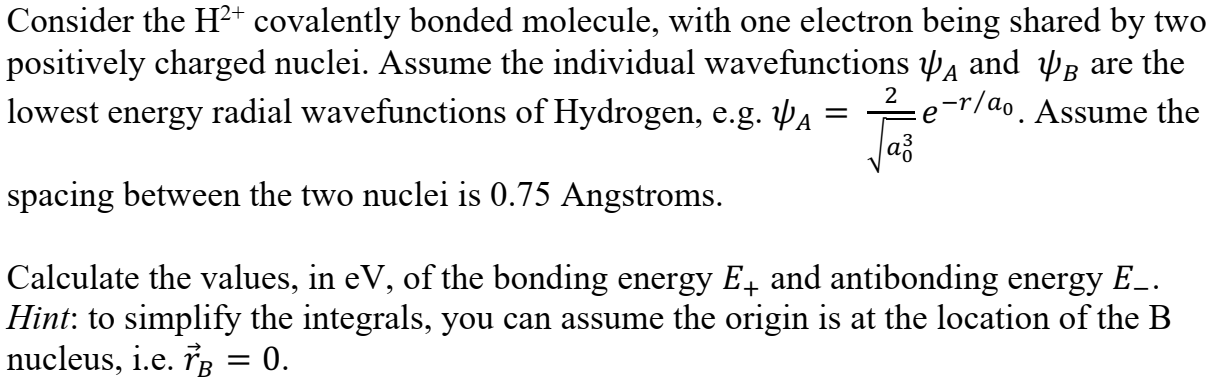
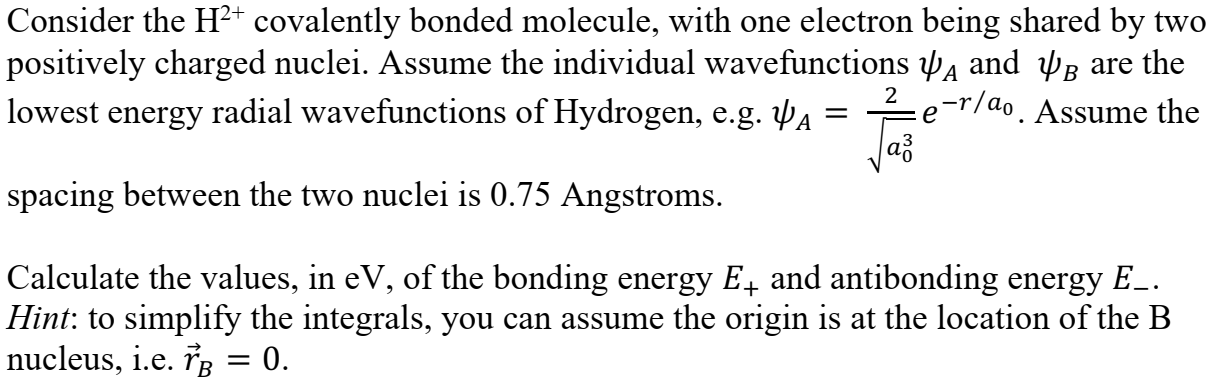
Consider the H2 covalently bonded molecule, with one electron being shared by two positively charged nuclei. Assume the individual wavefunctions w and Up are the lowest energy radial wavefunctions of Hydrogen, e.g. YA = 2 p-r/do . Assume the a3 spacing between the two nuclei is 0.75 Angstroms. Calculate the values, in ev, of the bonding energy E, and antibonding energy E_. Hint: to simplify the integrals, you can assume the origin is at the location of the B nucleus, i.e. TB = 0
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


