Question: d. Given that the alcohol is the limiting reactant and that all of the unknowns have the same molecular weight, calculate the % yield of
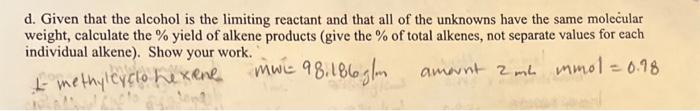
d. Given that the alcohol is the limiting reactant and that all of the unknowns have the same molecular weight, calculate the % yield of alkene products (give the % of total alkenes, not separate values for each individual alkene). Show your work. fomethilcuclohexene mwe 98.186glm amount 2mL mmol =0.98 d. Given that the alcohol is the limiting reactant and that all of the unknowns have the same molecular weight, calculate the % yield of alkene products (give the % of total alkenes, not separate values for each individual alkene). Show your work. fomethilcuclohexene mwe 98.186glm amount 2mL mmol =0.98
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


