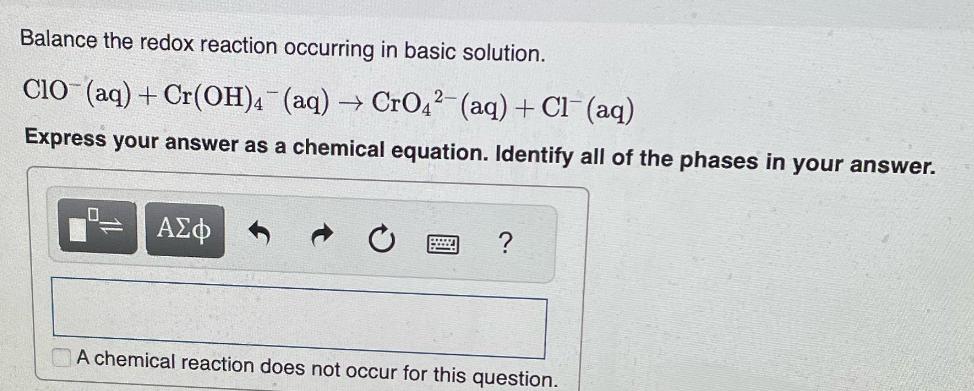
Question: Balance the redox reaction occurring in basic solution. CIO (aq) + Cr(OH)4 (aq) CrO42(aq) + Cl(aq) Express your answer as a chemical equation. Identify
Balance the redox reaction occurring in basic solution. CIO (aq) + Cr(OH)4 (aq) CrO42(aq) + Cl(aq) Express your answer as a chemical equation. Identify all of the phases in your answer. ? A chemical reaction does not occur for this question.
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts