Question: Dill & Bromberg, Problems 13.3 and 13.4 (a). Compute Kp, the pressure-based equilibrium constant for the dissociation of oxygen, O22O at T=3000K. The electronic ground-state
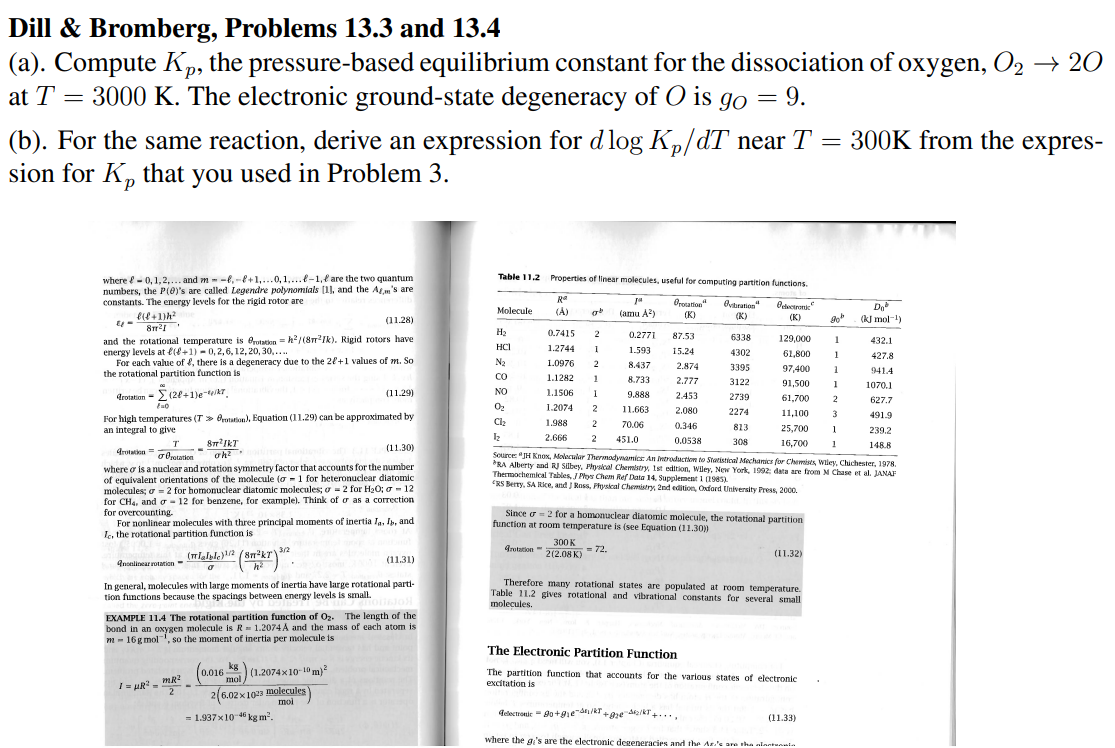
Dill \& Bromberg, Problems 13.3 and 13.4 (a). Compute Kp, the pressure-based equilibrium constant for the dissociation of oxygen, O22O at T=3000K. The electronic ground-state degeneracy of O is gO=9. (b). For the same reaction, derive an expression for dlogKp/dT near T=300K from the expression for Kp that you used in Problem 3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


