Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The purpose of this experiment is to synthesize dibenzalacetone (trans, trans-1, 5-diphenyl-1,4- pentadien-3-one) through the Aldol condensation of acetone with benzaldehyde. The synthesis begins
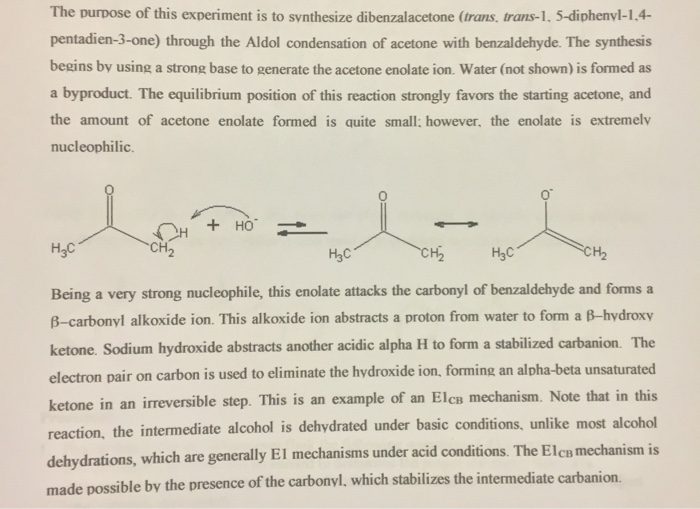
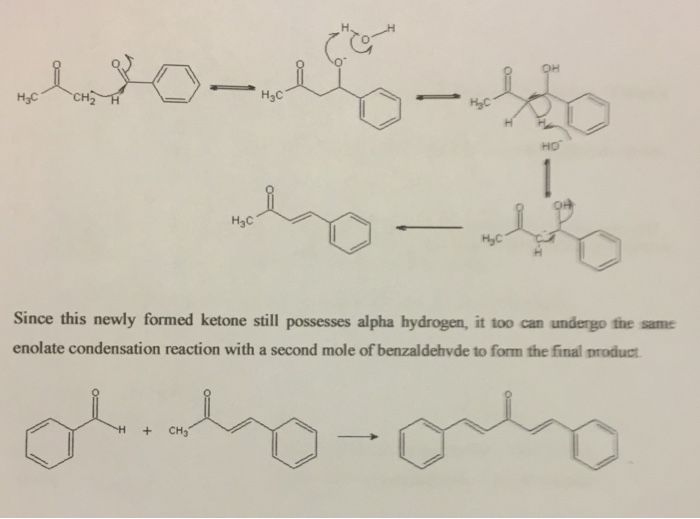
The purpose of this experiment is to synthesize dibenzalacetone (trans, trans-1, 5-diphenyl-1,4- pentadien-3-one) through the Aldol condensation of acetone with benzaldehyde. The synthesis begins by using a strong base to generate the acetone enolate ion. Water (not shown) is formed as a byproduct. The equilibrium position of this reaction strongly favors the starting acetone, and the amount of acetone enolate formed is quite small; however, the enolate is extremely nucleophilic. + HO HC -CH HC CH HC CH Being a very strong nucleophile, this enolate attacks the carbonyl of benzaldehyde and forms a B-carbonyl alkoxide ion. This alkoxide ion abstracts a proton from water to form a B-hydroxy ketone. Sodium hydroxide abstracts another acidic alpha H to form a stabilized carbanion. The electron pair on carbon is used to eliminate the hydroxide ion, forming an alpha-beta unsaturated ketone in an irreversible step. This is an example of an Elc mechanism. Note that in this reaction, the intermediate alcohol is dehydrated under basic conditions, unlike most alcohol dehydrations, which are generally El mechanisms under acid conditions. The ElcB mechanism is made possible by the presence of the carbonyl, which stabilizes the intermediate carbanion. -- HC HC HO hy-the HC HC Since this newly formed ketone still possesses alpha hydrogen, it too can undergo the same enolate condensation reaction with a second mole of benzaldehyde to form the final product. obily-oby CH
Step by Step Solution
★★★★★
3.49 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
H osf tho heat ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


