Question: Draw the missing lone pairs and label each lone pair as localized or delocalized. Also label the N and S hybridization state. Then overall, how
Draw the missing lone pairs and label each lone pair as localized or delocalized. Also label the N and S hybridization state. Then overall, how many pairs of pi electrons does the strucute have and is it aromatic, nonaromatic, or aniaromatc in the structure drawn? 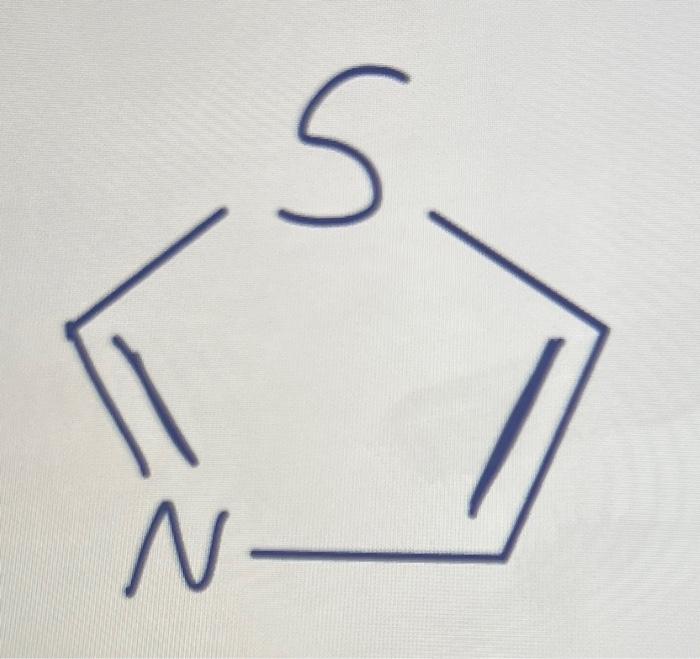

Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


