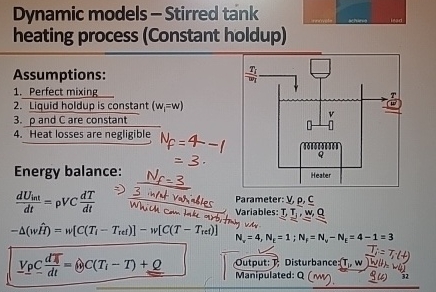
Question: Dynamic models - Stirred tank heating process ( Constant holdup ) Assumptions: Perfect mixing Liquid holdup is constant ) = ( w p and C
Dynamic models Stirred tank heating process Constant holdup
Assumptions:
Perfect mixing
Liquid holdup is constant
and are constant
Heat losses are negligible
Energy balance:
Parameter:
Which com take amb, Pariables:
;
eutput: Y Disturbance:
Manipulated:
detail out how we arrive the mass and energy balance equation given in the figure
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


