Question: (e) A solution containing 7.0 mass %C2H5OH is made by dilution of a stream containing 30 mass %C2H5OH with a stream of pure water. Calculate
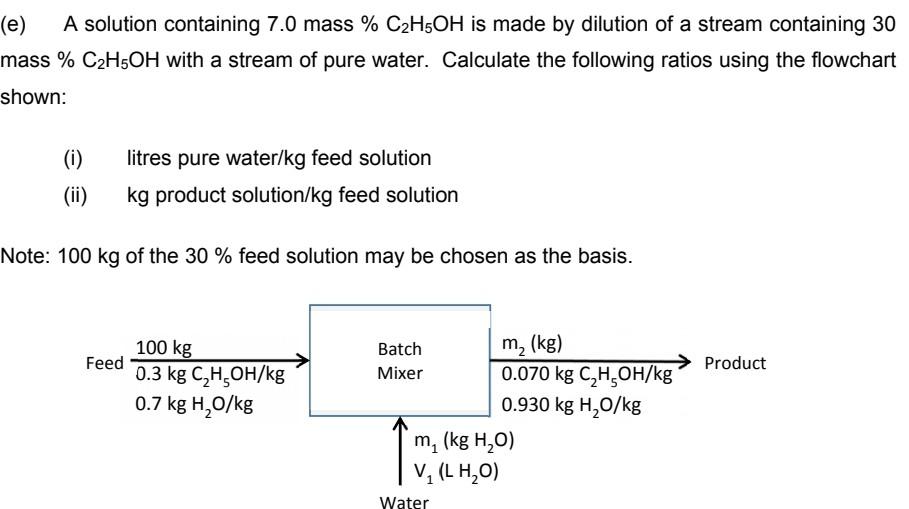
(e) A solution containing 7.0 mass %C2H5OH is made by dilution of a stream containing 30 mass %C2H5OH with a stream of pure water. Calculate the following ratios using the flowchart shown: (i) litres pure water/kg feed solution (ii) kg product solution/kg feed solution Note: 100kg of the 30% feed solution may be chosen as the basis
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


