Question: em Set: Chapter 6- Moles and Mass 1. How many moles of K+ ions are in 1.75 mol of K2SO4 2. How many calcium
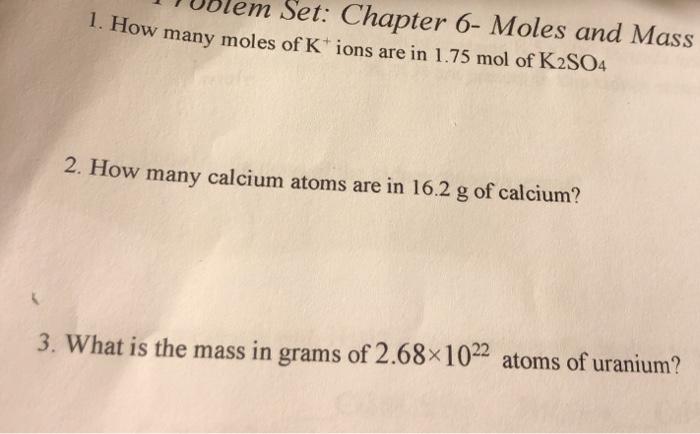
em Set: Chapter 6- Moles and Mass 1. How many moles of K+ ions are in 1.75 mol of K2SO4 2. How many calcium atoms are in 16.2 g of calcium? 3. What is the mass in grams of 2.68x1022 atoms of uranium?
Step by Step Solution
★★★★★
3.38 Rating (151 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

0 9 L Mole K 6825g 175 mol 39 gmol Mole K 175 moll Mol K50... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


