Question: Energy Balance A continuous flash separator separates a liquid inlet stream at 120C containing 40% benzene ( C6H6) and 60% toluene (C7H8) by mole. In
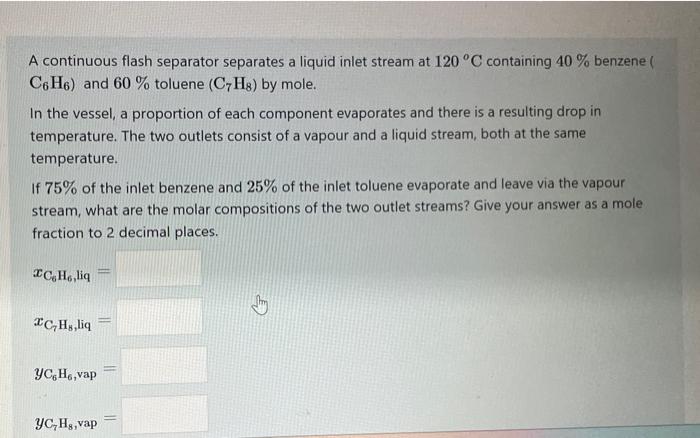
A continuous flash separator separates a liquid inlet stream at 120C containing 40% benzene ( C6H6) and 60% toluene (C7H8) by mole. In the vessel, a proportion of each component evaporates and there is a resulting drop in temperature. The two outlets consist of a vapour and a liquid stream, both at the same temperature. If 75% of the inlet benzene and 25% of the inlet toluene evaporate and leave via the vapour stream, what are the molar compositions of the two outlet streams? Give your answer as a mole fraction to 2 decimal places. xC6H6,iq=xC7H8,liq=yC6H6,vap=yC7H8,vap=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


