Question: Enter density values in g/mL to the nearest 0.001gmL. Include the unit using the symbol g/mL Enter temperafure values in C to the nearest 01C.
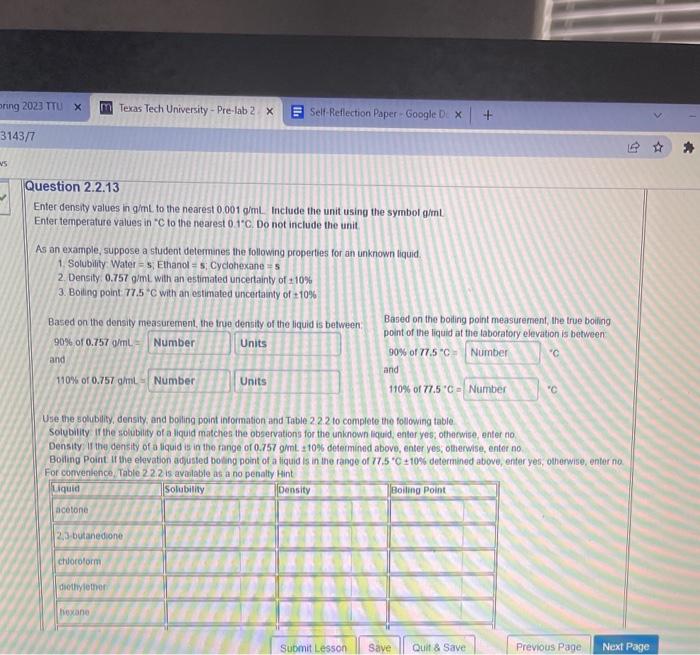
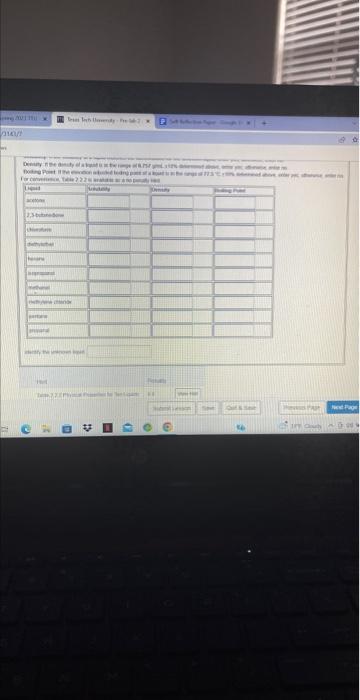
Enter density values in g/mL to the nearest 0.001gmL. Include the unit using the symbol g/mL Enter temperafure values in C to the nearest 01C. Do not include the unit. As an example, suppose a sludent detemines the following properties for an unknown liquid. 1. Solubility . Water =5; Ethanol =5;, Cyclohexane =5 2. Densily 0.757gmL with an estimated uncertainty of 10% 3. Boiling point 77.5C with an estimated uncertainty of 10% Based on the density measurement, the true density of the liquid is between: Based on the boling point measurement, the true boiling \begin{tabular}{l|l} 90% of 0.757gmL= & point of the liquid at the taboratory elevation is \\ and & 90% of 77.5C=m \\ 110% of 0.757g/mL & andC \\ 110% of 77.5C= \end{tabular} Use the solubility, density, and boiling point intormation and Table 2.2.2 to complete the following table. Solubility if the solubility of a liquid matches the observations for the unknown liquid, enter yes; ohnacwise, enter no. Density if the density of a liquid is in the fange of 0.757gmL=10% determined above, enter yes, obleewise, entet no. Boiling Point if the elevation ad,usted boting point of a tiquid is in the range of 77.5C10% determined above, enter yes, othenwise, enter no. For comvenlence Table 22.2 is available as a no penalty Hint Enter density values in g/mL to the nearest 0.001gmL. Include the unit using the symbol g/mL Enter temperafure values in C to the nearest 01C. Do not include the unit. As an example, suppose a sludent detemines the following properties for an unknown liquid. 1. Solubility . Water =5; Ethanol =5;, Cyclohexane =5 2. Densily 0.757gmL with an estimated uncertainty of 10% 3. Boiling point 77.5C with an estimated uncertainty of 10% Based on the density measurement, the true density of the liquid is between: Based on the boling point measurement, the true boiling \begin{tabular}{l|l} 90% of 0.757gmL= & point of the liquid at the taboratory elevation is \\ and & 90% of 77.5C=m \\ 110% of 0.757g/mL & andC \\ 110% of 77.5C= \end{tabular} Use the solubility, density, and boiling point intormation and Table 2.2.2 to complete the following table. Solubility if the solubility of a liquid matches the observations for the unknown liquid, enter yes; ohnacwise, enter no. Density if the density of a liquid is in the fange of 0.757gmL=10% determined above, enter yes, obleewise, entet no. Boiling Point if the elevation ad,usted boting point of a tiquid is in the range of 77.5C10% determined above, enter yes, othenwise, enter no. For comvenlence Table 22.2 is available as a no penalty Hint
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


