Question: Entropy vs Temperature for O2 200 4. Here is a picture of the molar entropy of oxygen (from Engel's book). Based on this graph, estimate
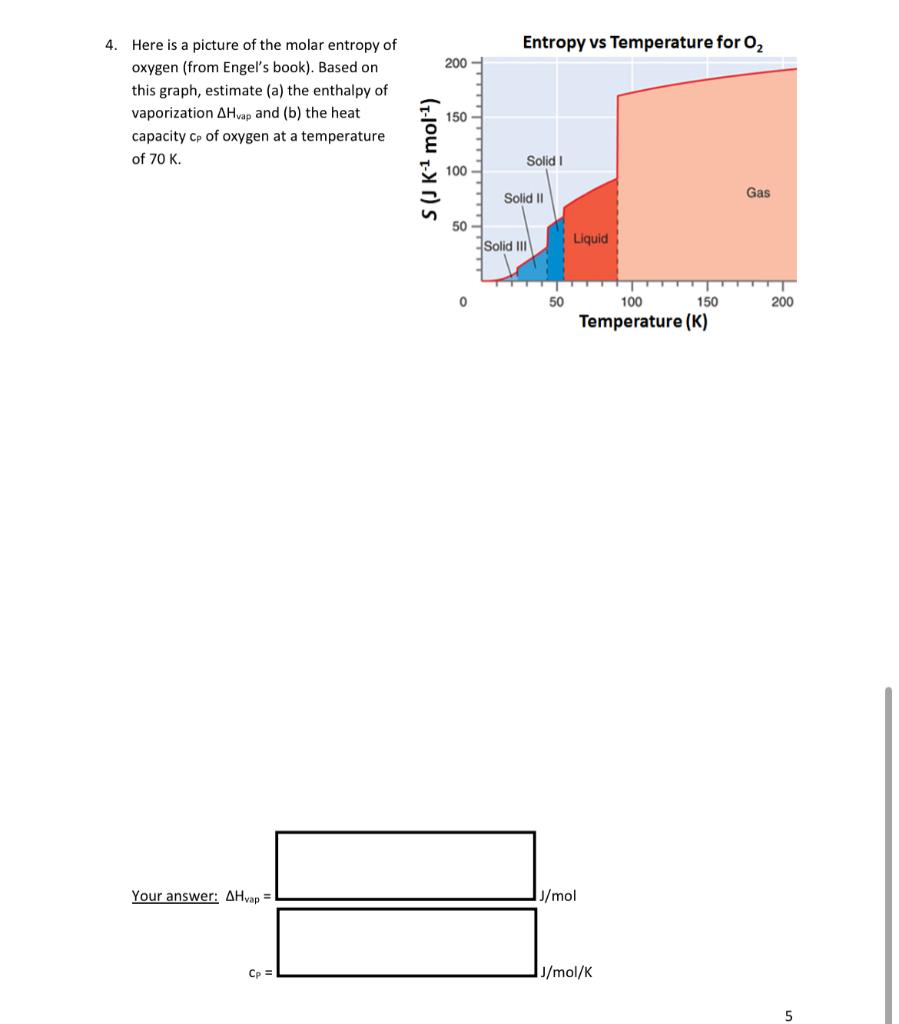
Entropy vs Temperature for O2 200 4. Here is a picture of the molar entropy of oxygen (from Engel's book). Based on this graph, estimate (a) the enthalpy of vaporization AH vap and (b) the heat capacity Cp of oxygen at a temperature of 70 K. 150 S(J K 1 mol-1) Solid 100 Solid 11 Gas 50 Solid III Liquid 0 50 200 100 150 Temperature (K) Your answer: AHvap = J/mol Cp = J/mol/K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


