Question: Equilibrium constant Solution 1 2 3 4 5 Absorbance 0.07 0.20 0.31 0.42 0.50 Initial molarity (= mol/L) Equilibrium Concentrations Fe3+ HSCN FeSCN+ Fe+
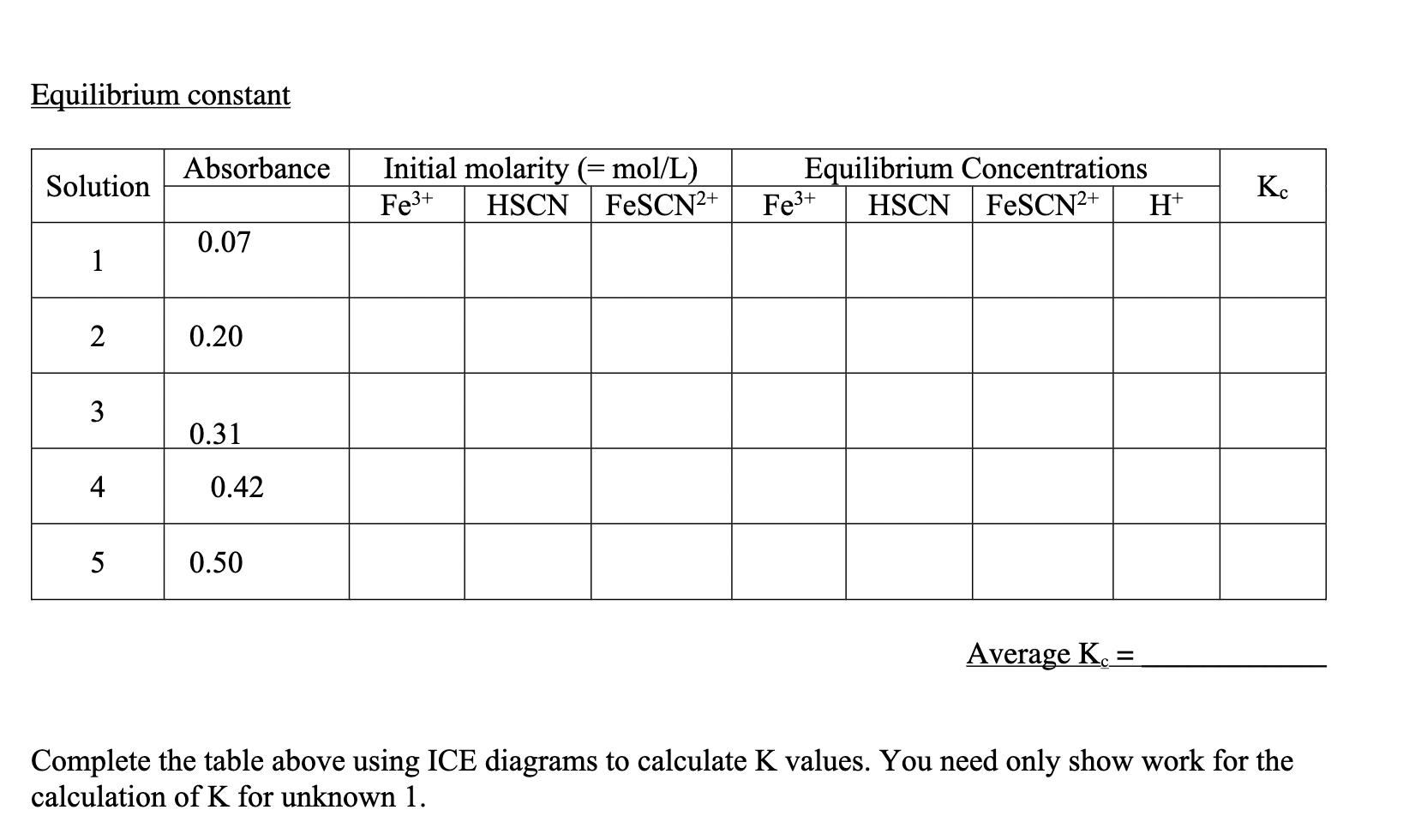
Equilibrium constant Solution 1 2 3 4 5 Absorbance 0.07 0.20 0.31 0.42 0.50 Initial molarity (= mol/L) Equilibrium Concentrations Fe3+ HSCN FeSCN+ Fe+ HSCN FeSCN+ H+ Average K. = Kc Complete the table above using ICE diagrams to calculate K values. You need only show work for the calculation of K for unknown 1.
Step by Step Solution
3.43 Rating (153 Votes )
There are 3 Steps involved in it
Solution Absorbance hitial molarity molL HSCN 000020 1 2 3 007 Average 620 031 642 050 Average Ko F3 ... View full answer

Get step-by-step solutions from verified subject matter experts


