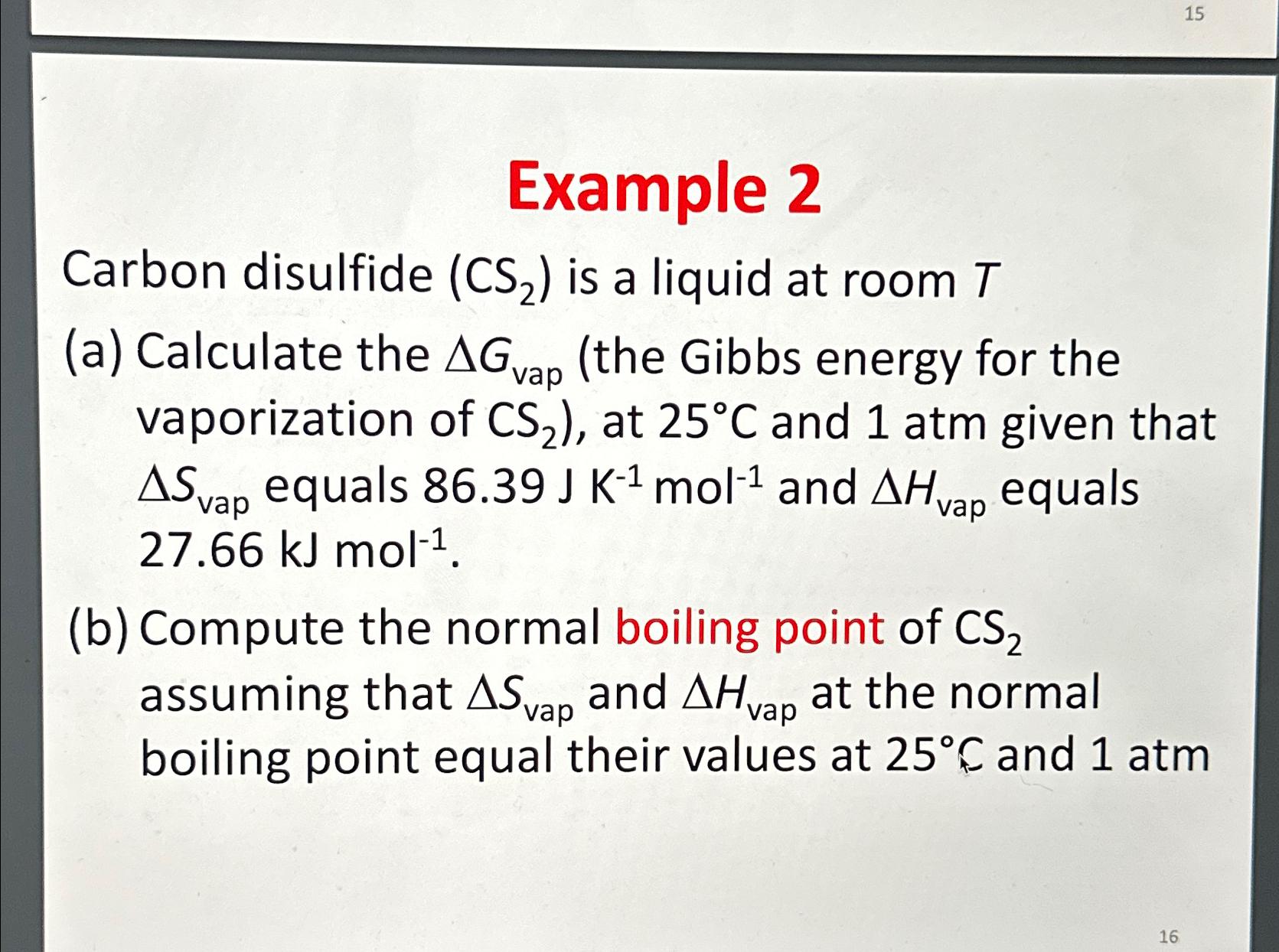
Question: Example 2 Carbon disulfide ( C S 2 ) is a liquid at room T ( a ) Calculate the G v a p (
Example
Carbon disulfide is a liquid at room
a Calculate the the Gibbs energy for the vaporization of at and atm given that equals and equals
b Compute the normal boiling point of assuming that and at the normal boiling point equal their values at and atm
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


