Question: A solid mixture weighing 0.548 5 g contained only ferrous ammonium sulfate hexahydrate and ferrous chloride hexahydrate. The sample was dissolved in 1 M
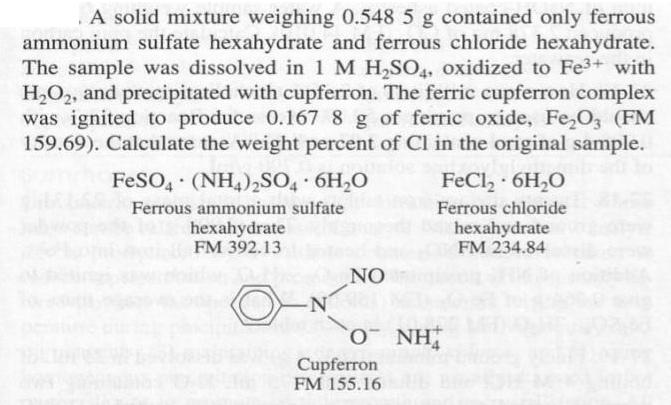
A solid mixture weighing 0.548 5 g contained only ferrous ammonium sulfate hexahydrate and ferrous chloride hexahydrate. The sample was dissolved in 1 M H,SO,, oxidized to Fe3+ with H,O2, and precipitated with cupferron. The ferric cupferron complex was ignited to produce 0.167 8 g of ferric oxide, Fe,O, (FM 159.69). Calculate the weight percent of Cl in the original sample. FeSO, (NH4)2SO, 6H,O FeCl, 6H,O Ferrous ammonium sulfate Ferrous chloride hexahydrate FM 392.13 hexahydrate FM 234.84 NO O NH Cupferron FM 155.16
Step by Step Solution
3.36 Rating (146 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


