Question: Exercise 9 Determine Ecell and Ecell based on the following half- reactions: VO+ + 2H+ + VO+ + HO Zn+ + 2e Zn Where:
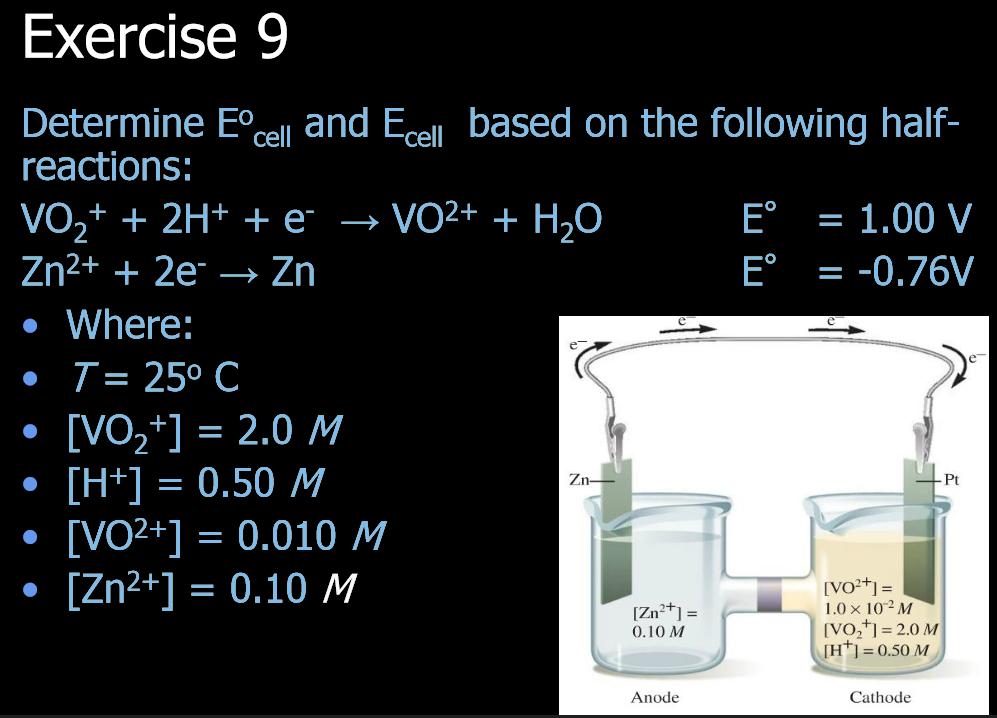
Exercise 9 Determine Ecell and Ecell based on the following half- reactions: VO+ + 2H+ + VO+ + HO Zn+ + 2e Zn Where: [H+] = 0.50 M T= 25 C [VO+] = 2.0 M 2 [VO+] = 0.010 M [Zn+] = 0.10 M Zn- [Zn+] = 0.10 M Anode E = 1.00 V E = -0.76V - Pt [VO+] = 1.0 10 M [VO+] = 2.0 M [H] = 0.50 M Cathode
Step by Step Solution
3.32 Rating (143 Votes )
There are 3 Steps involved in it
Answer Ans Given Hall cells V 2H e Zn12 overall 2 e ... View full answer

Get step-by-step solutions from verified subject matter experts


