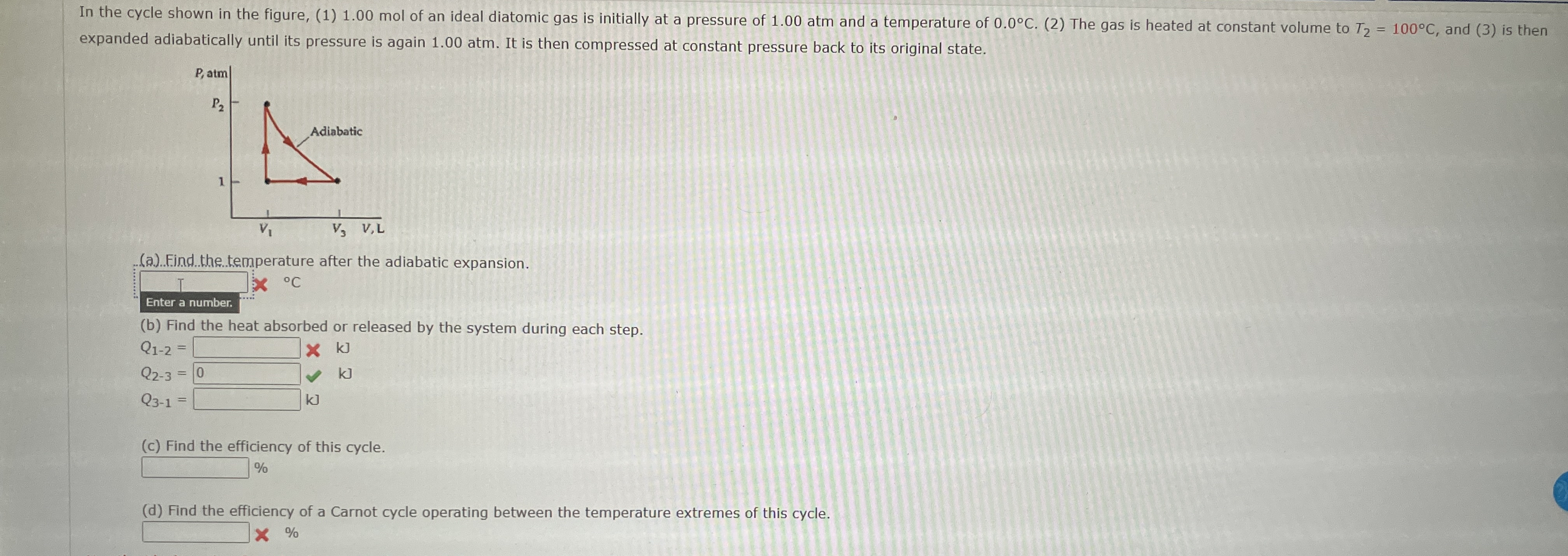
Question: expanded adiabatically until its pressure is again 1 . 0 0 atm . It is then compressed at constant pressure back to its original state.
expanded adiabatically until its pressure is again atm It is then compressed at constant pressure back to its original state.
a Find. the temperature after the adiabatic expansion.
Enter a number.
b Find the heat absorbed or released by the system during each step.
kJ
c Find the efficiency of this cycle.
d Find the efficiency of a Carnot cycle operating between the temperature extremes of this cycle.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


