Question: Explain the answer to a) and b), step by step very detailed please = Consider the elementary reaction of an organic molecule in an atmosphere
 Explain the answer to a) and b), step by step very detailed please
Explain the answer to a) and b), step by step very detailed please
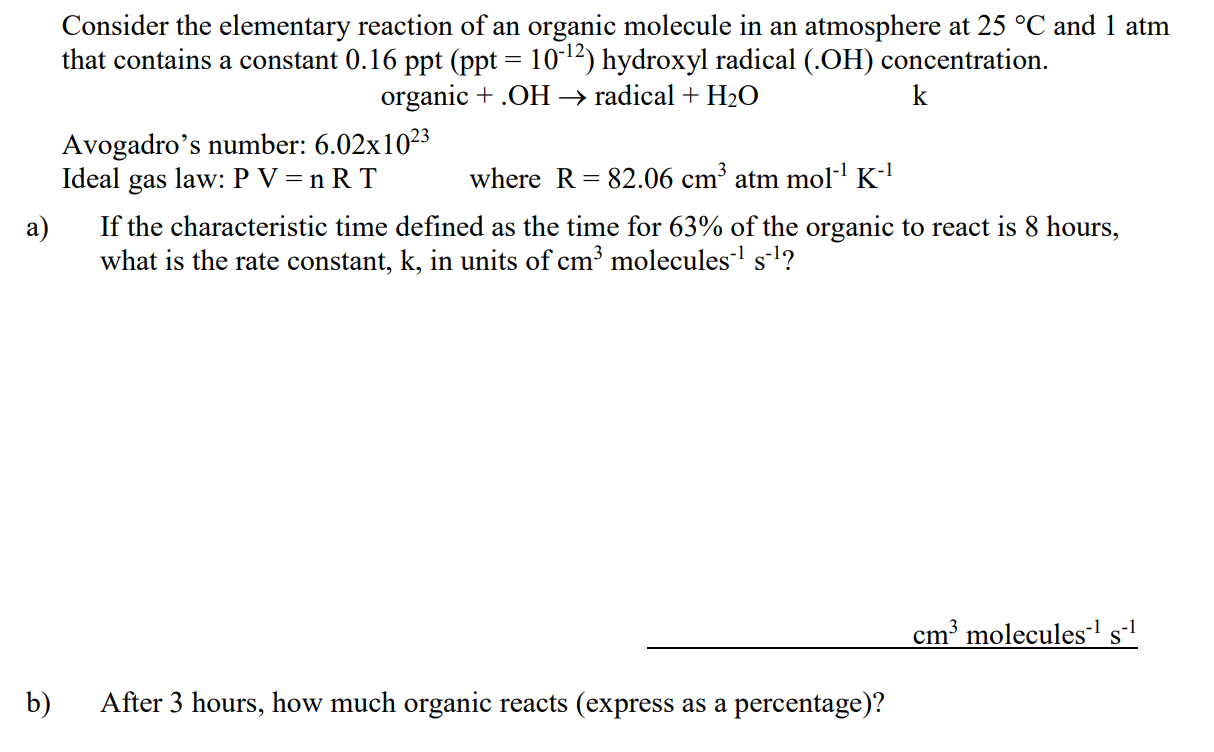
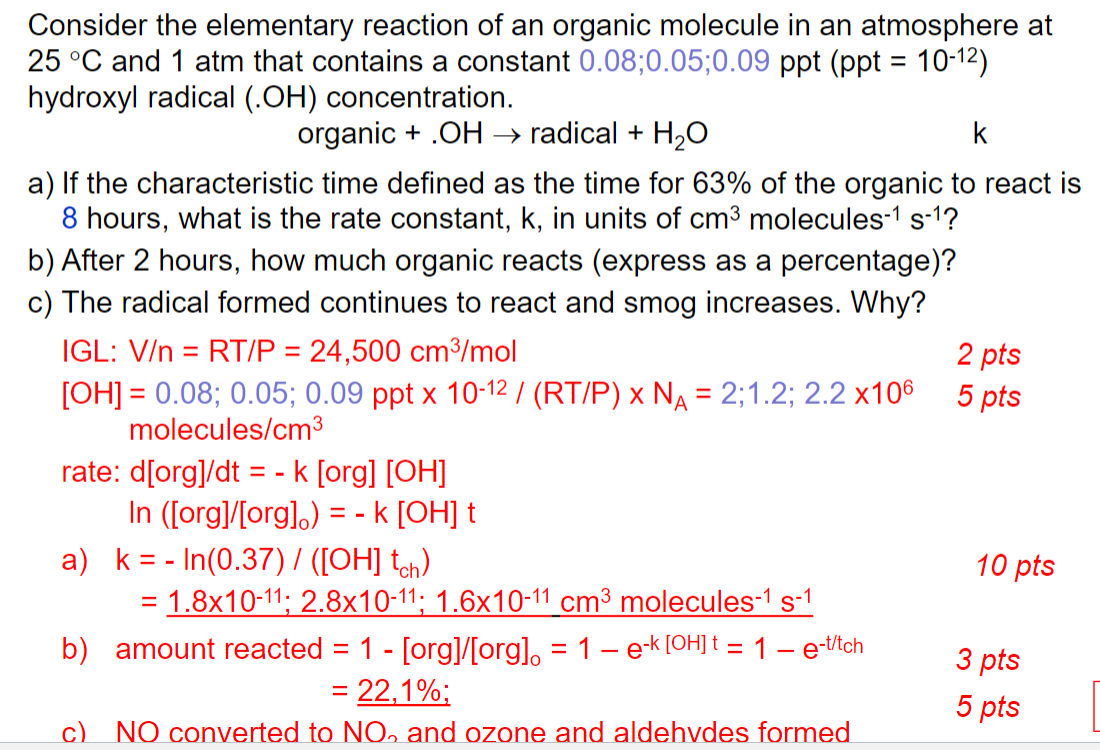
= Consider the elementary reaction of an organic molecule in an atmosphere at 25 C and 1 atm that contains a constant 0.16 ppt (ppt = 10-12) hydroxyl radical (.OH) concentration. organic +.OH radical + H2O Avogadro's number: 6.02x1023 Ideal gas law: P V=nRT where R = 82.06 cm3 atm mol-K-1 a) If the characteristic time defined as the time for 63% of the organic to react is 8 hours, what is the rate constant, k, in units of cm3 molecules 7 s-l? = cm? molecules-1 s-1 b) After 3 hours, how much organic reacts (express as a percentage)? - Consider the elementary reaction of an organic molecule in an atmosphere at 25 C and 1 atm that contains a constant 0.08;0.05;0.09 ppt (ppt = 10-12) hydroxyl radical (.OH) concentration. organic + .OH radical + H2O k a) If the characteristic time defined as the time for 63% of the organic to react is 8 hours, what is the rate constant, k, in units of cm3 molecules-1 s-1? b) After 2 hours, how much organic reacts (express as a percentage)? c) The radical formed continues to react and smog increases. Why? IGL: V = RT/P = 24,500 cm3/mol 2 pts [OH] = 0.08; 0.05; 0.09 ppt x 10-12 / (RT/P) x NA = 2;1.2; 2.2 x106 molecules/cm3 rate: d[org]/dt = - k [org] [OH] In ([org]/[org].) = - k [OH] t a) k = - In(0.37) / (OH] tch) 10 pts = 1.8x10-11; 2.8x10-11; 1.6x10-11 cm3 molecules-1 s-1 b) amount reacted = 1 - [org]/[orgl. = 1 e-k [OH] t = 1 e-titch = 22,1%; 5 pts c) NO converted to NO, and ozone and aldehydes formed 5 pts - - = 3 pts
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


