Question: Fill out the chart below using the Hion concentration to determine the pH and the pH to determine whether the solution is an acid, a
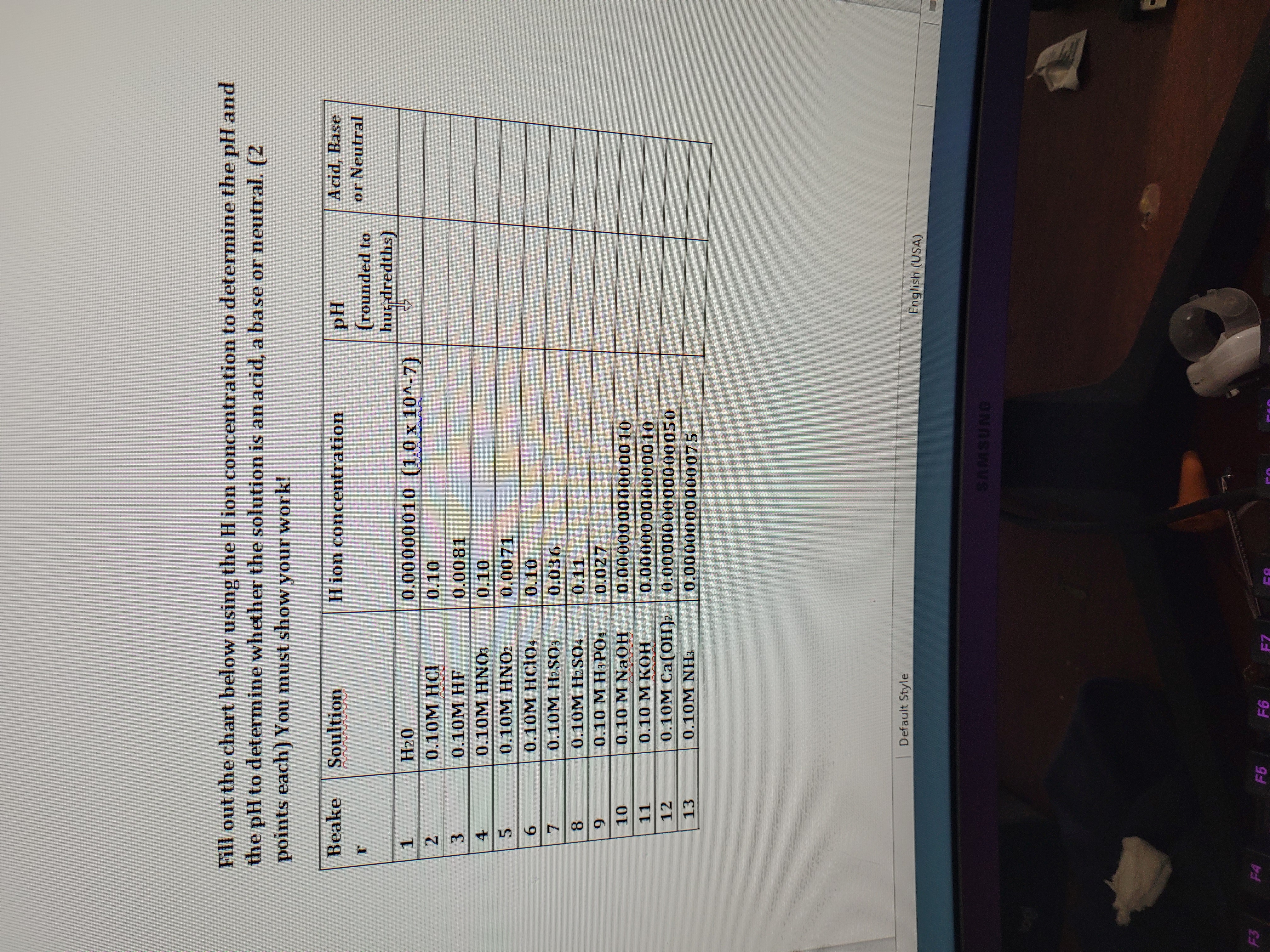
Fill out the chart below using the Hion concentration to determine the pH and the pH to determine whether the solution is an acid, a base or neutral. (2 points each) You must show your work! Beake Soultion H ion concentration PH Acid, Base (rounded to or Neutral hur dredths) 1 H20 0.00000010 (1.0 x 104-7) IN 0.10M HCI 0.10 3 0.10M HF 0.0081 4 0.10M HNO: 0.10 5 0.10M HNO 0.0071 6 0.10M HCIO4 0.10 7 0.10M H2SO3 0.036 8 0.10M H2SO4 0.11 9 0.10 M Ha PO4 0.027 10 0.10 M NaOH 0.00000000000010 11 0.10 M KOH 0.00000000000010 12 0.10M Ca (OH )2 0.000000000000050 13 0.10M NH3 0.0000000000075 Default Style English (USA) SAMSUNG F3 F4 F5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


