Question: final answer Consider the reaction corresponding to a voltaic cell and its standard cell potential. Zn(s)+Cu2+(aq)Cu(s)+Zn2+(aq)Ecell=1.1032V What is the cell potential for a cell with

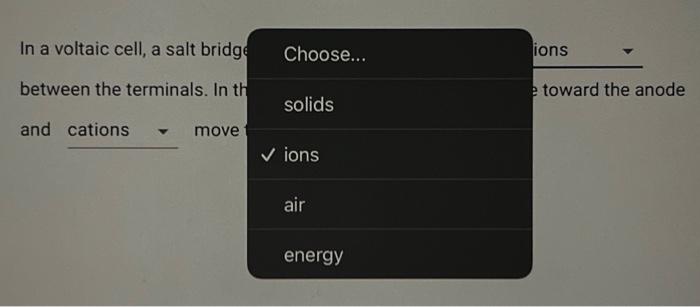
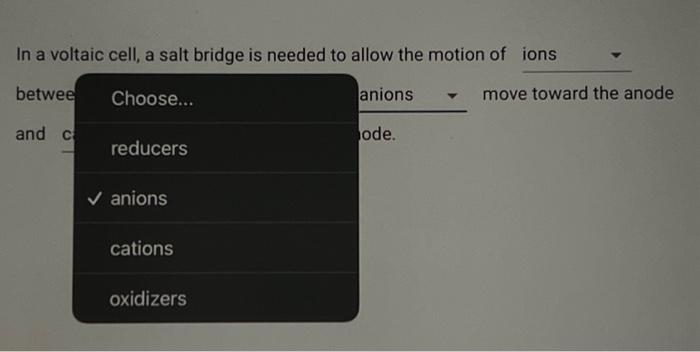
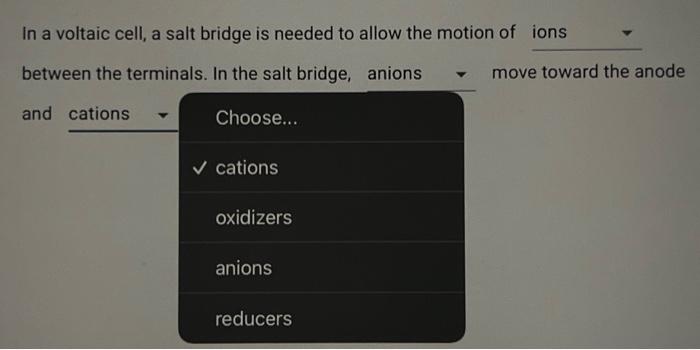

Consider the reaction corresponding to a voltaic cell and its standard cell potential. Zn(s)+Cu2+(aq)Cu(s)+Zn2+(aq)Ecell=1.1032V What is the cell potential for a cell with a 2.954M solution of Zn2+(aq) and 0.1940 M solution of Cu2+(aq) at 404.9K ? Type answer: In a voltaic cell, a salt bridg Choose... ions between the terminals. In th and solids toward the ano ions air energy In a voltaic cell, a salt bridge is needed to allow the motion of In a voltaic cell, a salt bridge is needed to allow the motion of between the terminals. In the salt bridge, anions move toward the anode In a voltaic cell, a salt bridge is needed to allow the motion of between the terminals. In the salt bridge, move toward the anode and move toward the cathode
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


