Question: find at least 3 errors in the given questions Intermolecular Forces, Phase Changes, and Solutions Solved Problems 1. One of the compounds shown here is
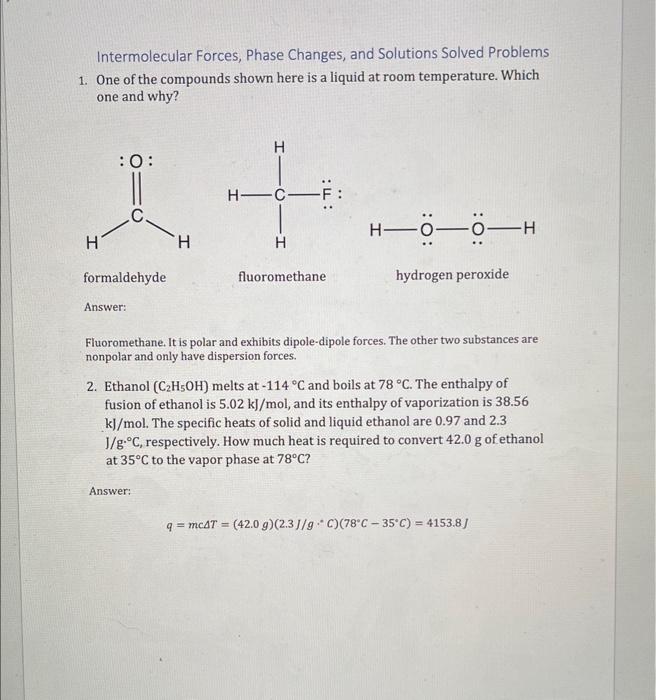
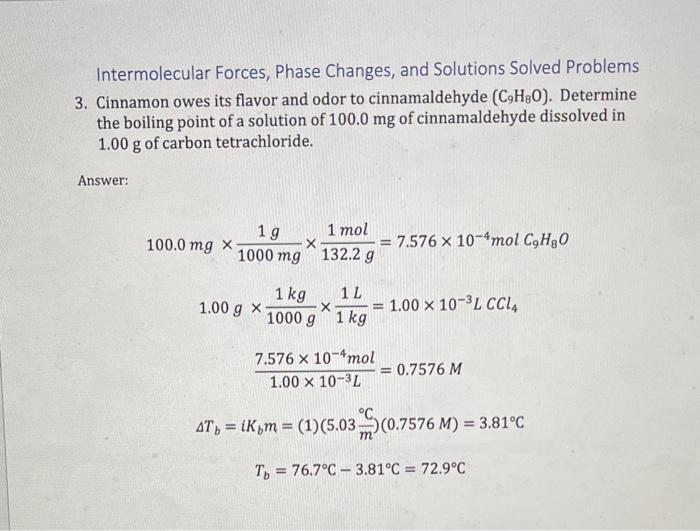
Intermolecular Forces, Phase Changes, and Solutions Solved Problems 1. One of the compounds shown here is a liquid at room temperature. Which one and why? Fluoromethane. It is polar and exhibits dipole-dipole forces. The other two substances are nonpolar and only have dispersion forces. 2. Ethanol (C2H5OH) melts at 114C and boils at 78C. The enthalpy of fusion of ethanol is 5.02kJ/mol, and its enthalpy of vaporization is 38.56 kj/mol. The specific heats of solid and liquid ethanol are 0.97 and 2.3 J/gC, respectively. How much heat is required to convert 42.0g of ethanol at 35C to the vapor phase at 78C ? Answer: q=mcT=(42.0g)(2.3J/gC)(78C35C)=4153.8J Intermolecular Forces, Phase Changes, and Solutions Solved Problems 3. Cinnamon owes its flavor and odor to cinnamaldehyde (C9H8O). Determine the boiling point of a solution of 100.0mg of cinnamaldehyde dissolved in 1.00g of carbon tetrachloride. Answer: 100.0mg1000mg1g132.2g1mol=7.576104molC9H8O1.00g1000g1kg1kg1L=1.00103LCCl41.00103L7.576104mol=0.7576MTb=iKbm=(1)(5.03mC)(0.7576M)=3.81CTb=76.7C3.81C=72.9C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


