Question: For a K + - C l - ion pair, attractive and repulsive energies E A and E R , respectively, depend on the distance
For a ion pair, attractive and repulsive energies and respectively, depend on the distance between the ions according to
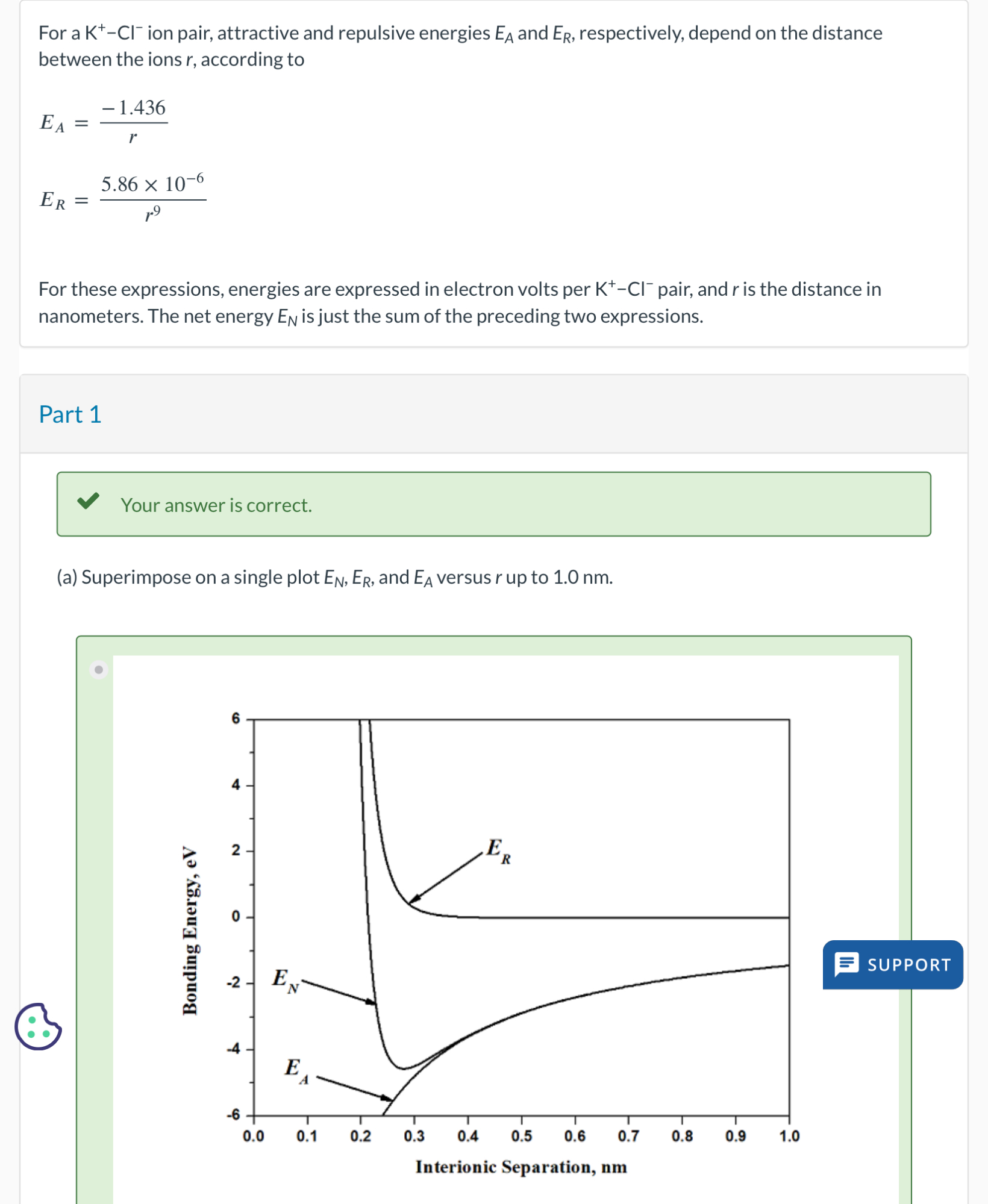
For these expressions, energies are expressed in electron volts per pair, and is the distance in nanometers. The net energy is just the sum of the preceding two expressions.
b On the basis of this plot, determine i the equilibrium spacing ro between the Kin nm and Cl ions, and ii the magnitude of the bonding energy Eo between the two ions in eV
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


