Question: For Chemical Engineering Separations: 1. Wankat 17.D5 [ Answer Key: a) 3.4/atm, b) A300m2 ] D5. We are using a cellulose acetate RO membrane to
For Chemical Engineering Separations:
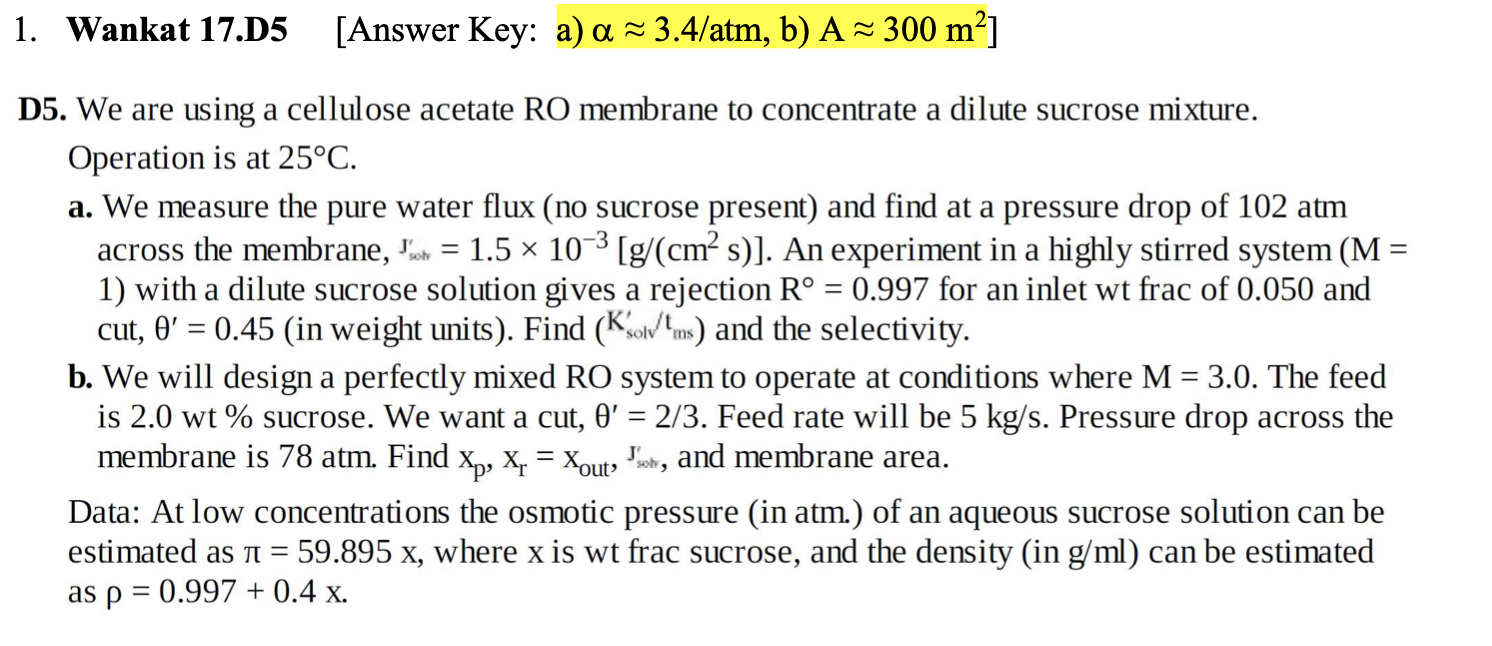
1. Wankat 17.D5 [ Answer Key: a) 3.4/atm, b) A300m2 ] D5. We are using a cellulose acetate RO membrane to concentrate a dilute sucrose mixture. Operation is at 25C. a. We measure the pure water flux (no sucrose present) and find at a pressure drop of 102atm across the membrane, sotj0it=1.5103[g/(cm2s)]. An experiment in a highly stirred system (M= 1) with a dilute sucrose solution gives a rejection R=0.997 for an inlet wt frac of 0.050 and cut, =0.45 (in weight units). Find (Ksolv/tms) and the selectivity. b. We will design a perfectly mixed RO system to operate at conditions where M=3.0. The feed is 2.0wt% sucrose. We want a cut, =2/3. Feed rate will be 5kg/s. Pressure drop across the membrane is 78atm. Find xp,xr=xoutsmit, and membrane area. Data: At low concentrations the osmotic pressure (in atm.) of an aqueous sucrose solution can be estimated as =59.895x, where x is wt frac sucrose, and the density (in g/ml ) can be estimated as =0.997+0.4x. 1. Wankat 17.D5 [ Answer Key: a) 3.4/atm, b) A300m2 ] D5. We are using a cellulose acetate RO membrane to concentrate a dilute sucrose mixture. Operation is at 25C. a. We measure the pure water flux (no sucrose present) and find at a pressure drop of 102atm across the membrane, sotj0it=1.5103[g/(cm2s)]. An experiment in a highly stirred system (M= 1) with a dilute sucrose solution gives a rejection R=0.997 for an inlet wt frac of 0.050 and cut, =0.45 (in weight units). Find (Ksolv/tms) and the selectivity. b. We will design a perfectly mixed RO system to operate at conditions where M=3.0. The feed is 2.0wt% sucrose. We want a cut, =2/3. Feed rate will be 5kg/s. Pressure drop across the membrane is 78atm. Find xp,xr=xoutsmit, and membrane area. Data: At low concentrations the osmotic pressure (in atm.) of an aqueous sucrose solution can be estimated as =59.895x, where x is wt frac sucrose, and the density (in g/ml ) can be estimated as =0.997+0.4x
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


