Question: For multi-electron systems things get more complex. For example, by analogy to orbital angular momentum we indicated the spin angular momentum obeys S^2spin=S(S+1)2spin where S^2=iS^i2
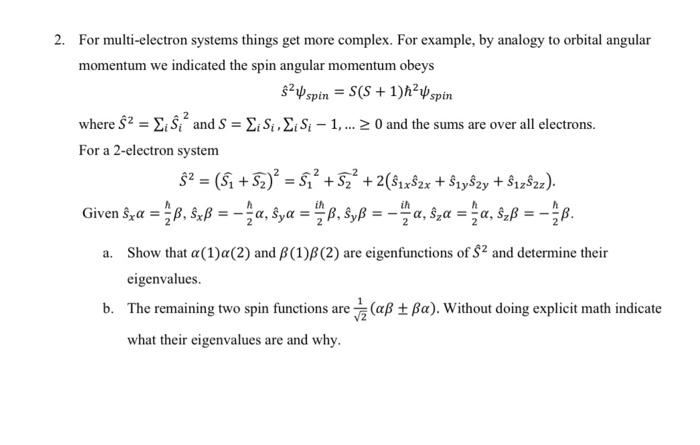
For multi-electron systems things get more complex. For example, by analogy to orbital angular momentum we indicated the spin angular momentum obeys S^2spin=S(S+1)2spin where S^2=iS^i2 and S=iSi,iSi1,0 and the sums are over all electrons. For a 2-electron system S^2=(S1+S2)2=S12+S22+2(S^1xS^2x+s^1ys^2y+s^1zS^2z) Given s^x=2h,s^x=2h,s^y=2i,s^y=2i,s^z=2h,s^z=2h. a. Show that (1)(2) and (1)(2) are eigenfunctions of S2 and determine their eigenvalues. b. The remaining two spin functions are 21(). Without doing explicit math indicate what their eigenvalues are and why
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


