Question: For the galvanic cell reaction, expressed below using shorthand notation, what half-reaction occurs at the cathode? Ba(s) Ba2+(aq) || Cd2+(aq) | Cd(s) Ba(s) Ba2+(aq)
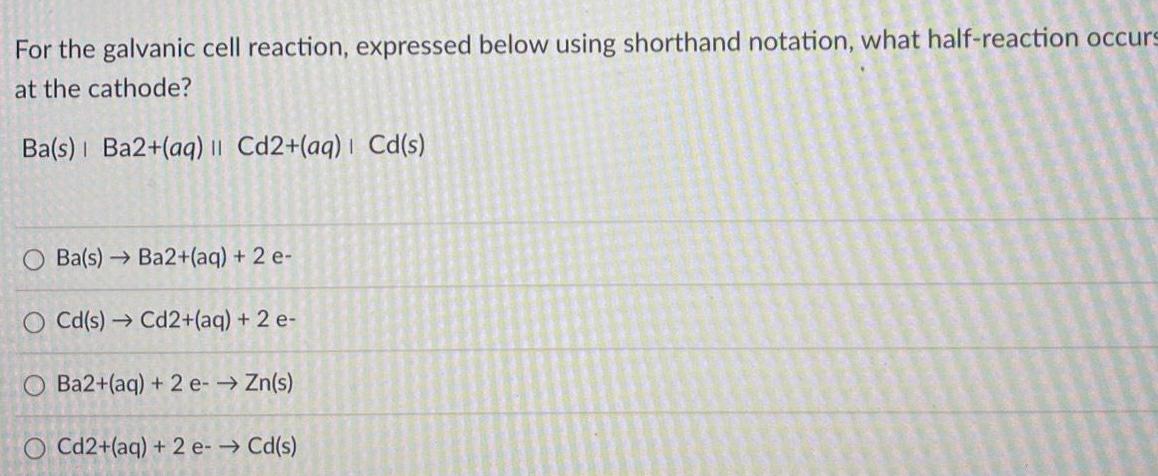
For the galvanic cell reaction, expressed below using shorthand notation, what half-reaction occurs at the cathode? Ba(s) Ba2+(aq) || Cd2+(aq) | Cd(s) Ba(s) Ba2+(aq) + 2 e- O Cd(s) Cd2+(aq) + 2 e- O Ba2+(aq) + 2e- Zn(s) O Cd2+(aq) + 2 e- Cd(s)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


