Question: For the liquid phase reaction AB, ra = -KFCA + kbCB = Starting from the mole balance for a CSTR, find the space time (To)
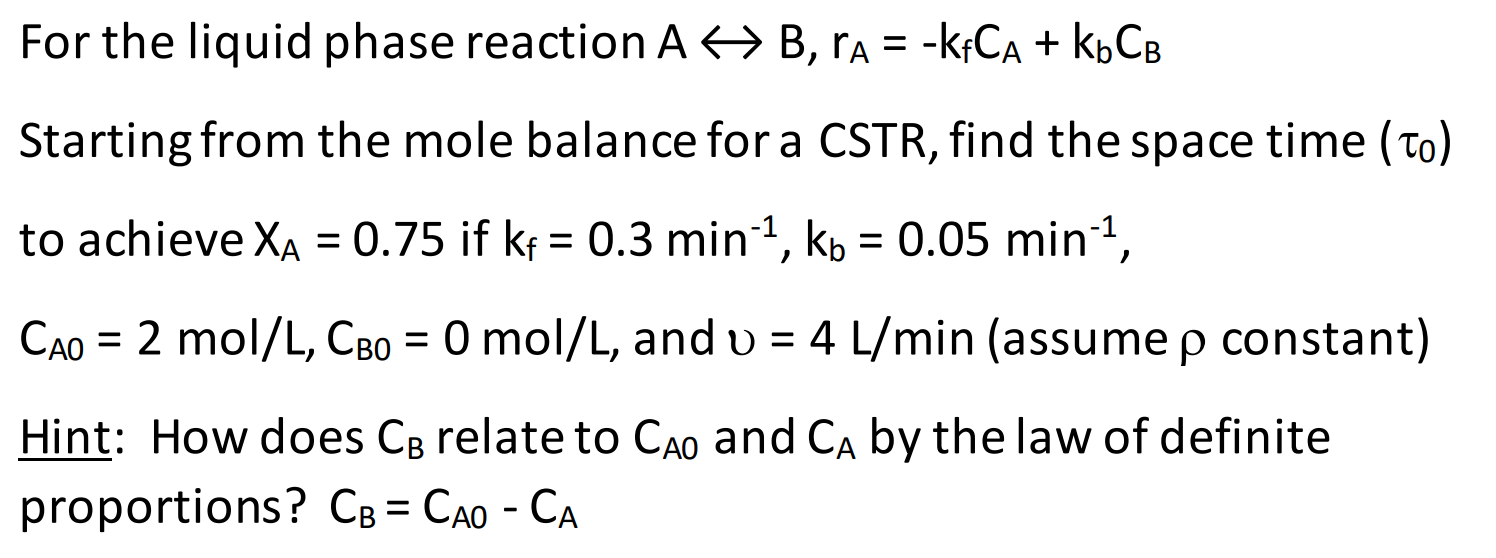
For the liquid phase reaction AB, ra = -KFCA + kbCB = Starting from the mole balance for a CSTR, find the space time (To) to achieve XA = 0.75 if kp = 0.3 min-1, kb = 0.05 min-1, = = = Cao = 2 mol/L, CBo = 0 mol/L, and v = 4 L/min (assume p constant) = = Hint: How does CB relate to Cao and CA by the law of definite proportions? CB = CAO - CA
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


