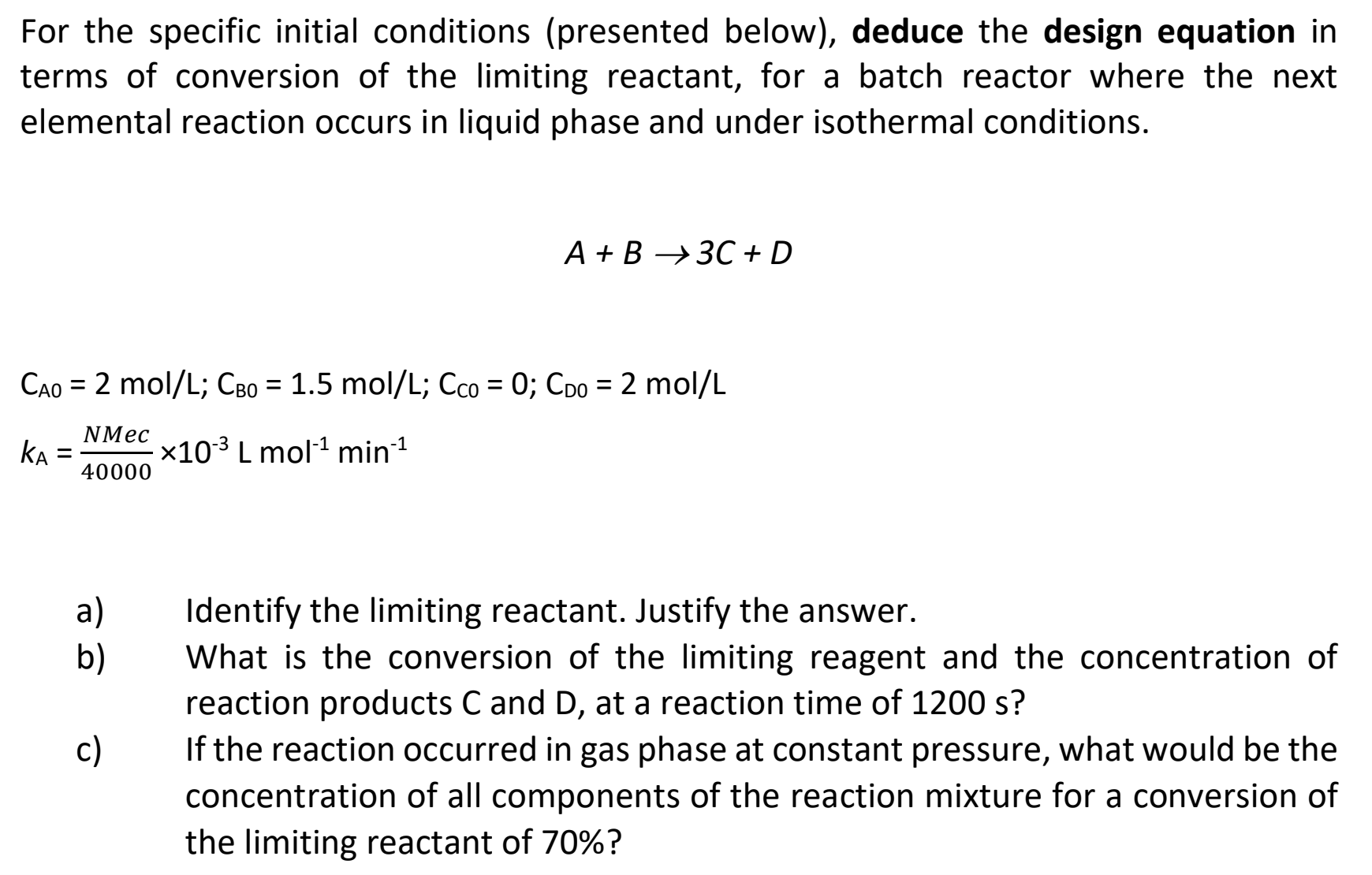
Question: For the specific initial conditions ( presented below ) , deduce the design equation in terms of conversion of the limiting reactant, for a batch
For the specific initial conditions presented below deduce the design equation in
terms of conversion of the limiting reactant, for a batch reactor where the next
elemental reaction occurs in liquid phase and under isothermal conditions.
;;;
a Identify the limiting reactant. Justify the answer.
b What is the conversion of the limiting reagent and the concentration of
reaction products and at a reaction time of
c If the reaction occurred in gas phase at constant pressure, what would be the
concentration of all components of the reaction mixture for a conversion of
the limiting reactant of
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


