Question: Four equal volume reactors connected in series and with continuous stirring under isothermal conditions is working. 1st Order (-rA=kCA) A B reaction takes place in
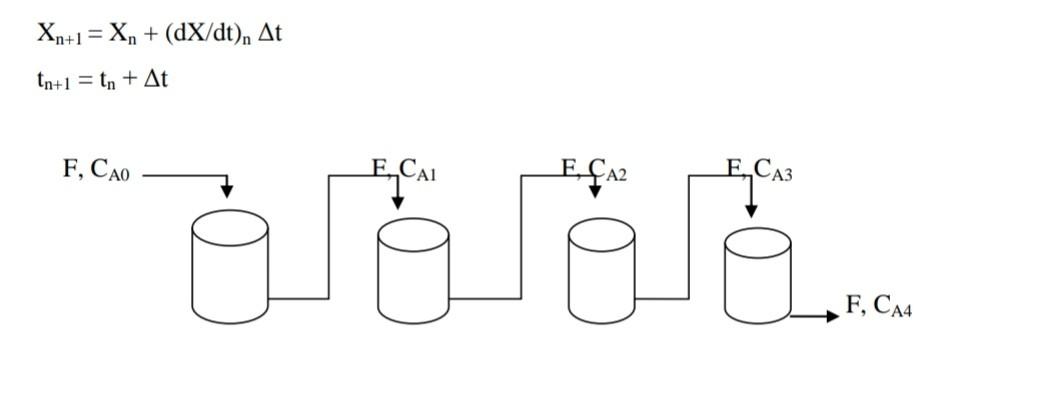
Four equal volume reactors connected in series and with continuous stirring under isothermal conditions is working. 1st Order (-rA=kCA) A B reaction takes place in the reactors. The volumetric flow rates of the flows entering and leaving the tanks are equal. In the initial conditions CA1(0)=0.6kmolA/m3 , CA2(0)=0.5kmolA/m3 , CA3(0)=0.3kmolA/m3 and CA4(0)=0.2kmolA/m3 Stop. The concentration of A entering the first tank is constant and CAo=1.8 kmolA/m3 Stop. reaction speed constant k=0.5min-1 and the V/F ratio is 2 min. Find the concentration of A in the tanks after 0.6 minutes using the Euler method, take t=0.2min.
Xn+1=Xn+(dX/dt)nttn+1=tn+t
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


