Question: From the following data plot, calculate the activation energy, Ea, for the reaction. How am I supposed to do that? I don't even know what
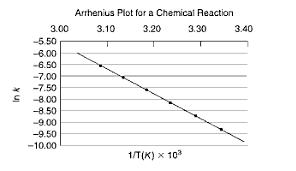
From the following data plot, calculate the activation energy, Ea, for the reaction.
How am I supposed to do that? I don't even know what those points are because they aren't directly at 3.10, etc, it's all aguess...
3.00 -5.50 -6.00 -6.50 -7.00 -7.50 S -8.00 -8.50 -9.00 -9.50 -10.00 Arrhenius Plot for a Chemical Reaction 3.10 3.20 3.30 1/T(K) x 10 3.40
Step by Step Solution
3.51 Rating (168 Votes )
There are 3 Steps involved in it
Answer us K A EART e log K logA EA R dd ... View full answer

Get step-by-step solutions from verified subject matter experts


