Question: Given the balanced equation below. Fe2O3(s)+3CO(g)2Fe(s)+3CO2(g)Hnnn=23.0kJ Determine whether each statement is true or false using the provided drop-down menus. 1) The standard enthalpy of formation
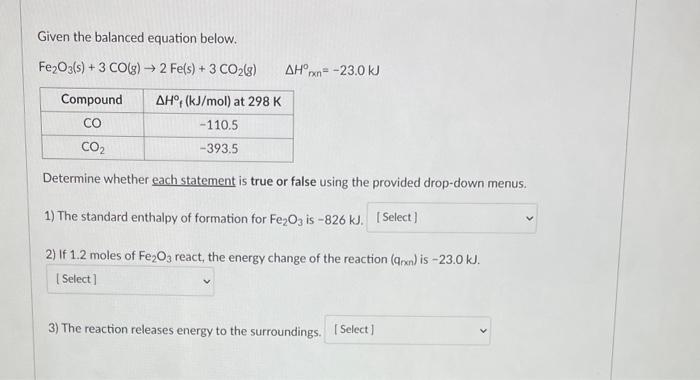
Given the balanced equation below. Fe2O3(s)+3CO(g)2Fe(s)+3CO2(g)Hnnn=23.0kJ Determine whether each statement is true or false using the provided drop-down menus. 1) The standard enthalpy of formation for Fe2O3 is 826kJ. 2) If 1.2 moles of Fe2O3 react, the energy change of the reaction (qnxn) is 23.0kJ. 3) The reaction releases energy to the surroundings
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


