Question: Given the data below, determine the experimental rate law and rate constant (k) for the reaction: H2SeO3(aq)+6I(aq)+4H+(aq)Se(s)+2I3(aq)+3H2O(l)
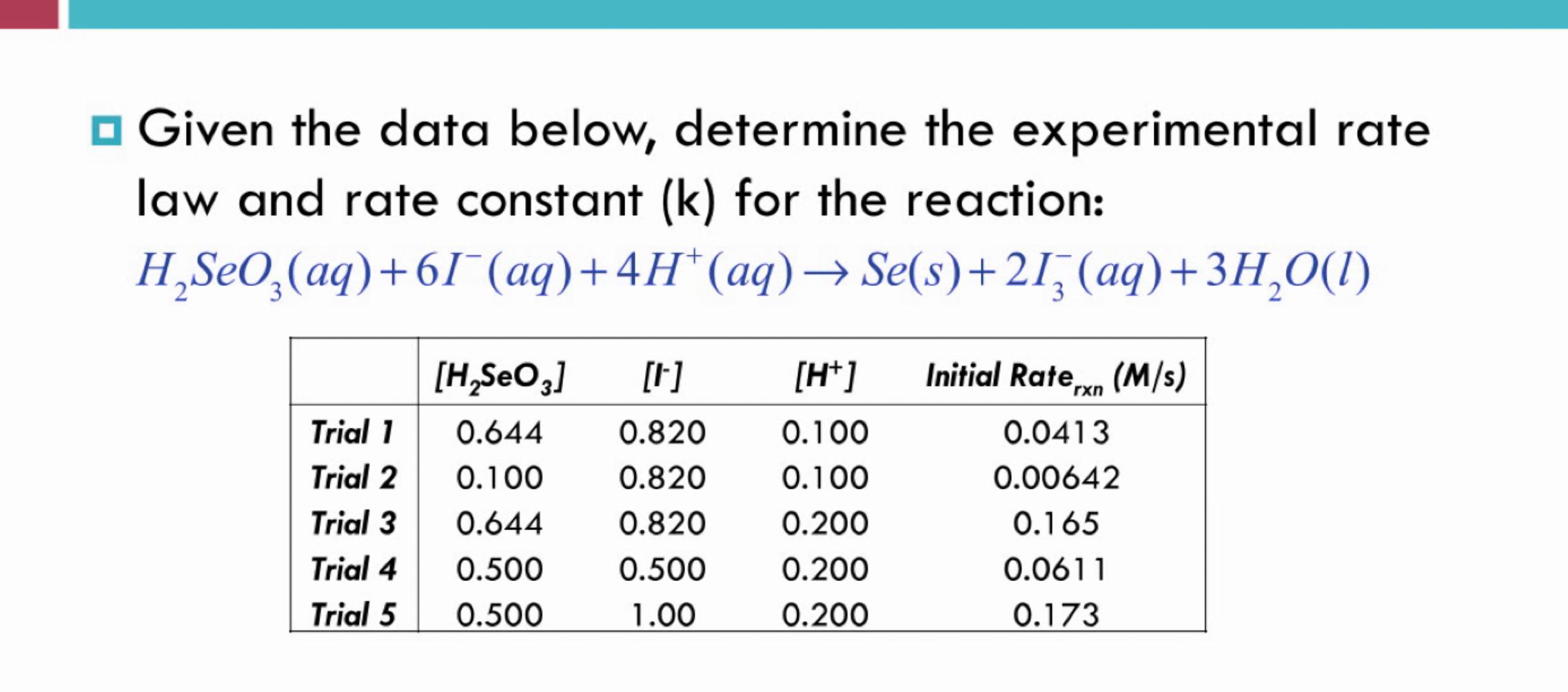
Given the data below, determine the experimental rate law and rate constant (k) for the reaction: H2SeO3(aq)+6I(aq)+4H+(aq)Se(s)+2I3(aq)+3H2O(l)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


