Question: = Given the elementary liquid phase reaction A+B C with ka = 0.35 L/mol.h. a. Calculate the concentration of species A after 3 h in
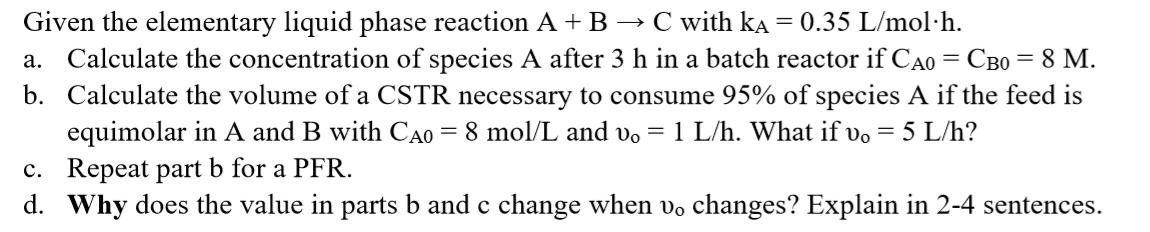
= Given the elementary liquid phase reaction A+B C with ka = 0.35 L/mol.h. a. Calculate the concentration of species A after 3 h in a batch reactor if Cao = CBo = 8 M. b. Calculate the volume of a CSTR necessary to consume 95% of species A if the feed is equimolar in A and B with Cao = 8 mol/L and v. = 1 L/h. What if vo = 5 L/h? c. Repeat part b for a PFR. d. Why does the value in parts b and c change when vo changes? Explain in 2-4 sentences. =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


