Question: Given the reactant amounts specified in each chemical equation, determine the amount in moles of excess reactant that remains. (a) HCl+NaOHNaCl+H2O 2.4molHCl and 2.5molNaOH amount
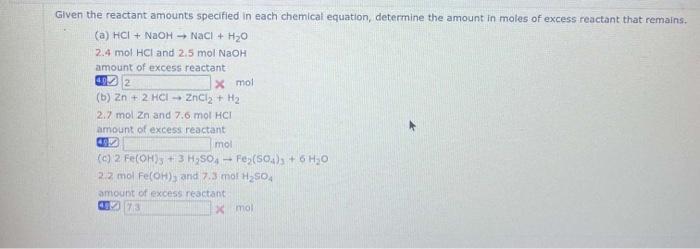
Given the reactant amounts specified in each chemical equation, determine the amount in moles of excess reactant that remains. (a) HCl+NaOHNaCl+H2O 2.4molHCl and 2.5molNaOH amount of excess reactant an. x mol (b) Zn+2HClZnCl2+H2 2.7 molZn and 7.6molHCI amount of excess reactant. . mol (c) 2FecOH)3+3H2SO4Fe2(SO4)3+6H2O 2.2molFe(OH)2 and 7.3molH2SO4 amount of excess reactant: ]. xmol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


