Question: H OH H=CHCH (1) + 30=0 (9) ) 3 + (9) 2 (9) H HH oxygen carbon dioxide water ethanol si Ethanol from corn is
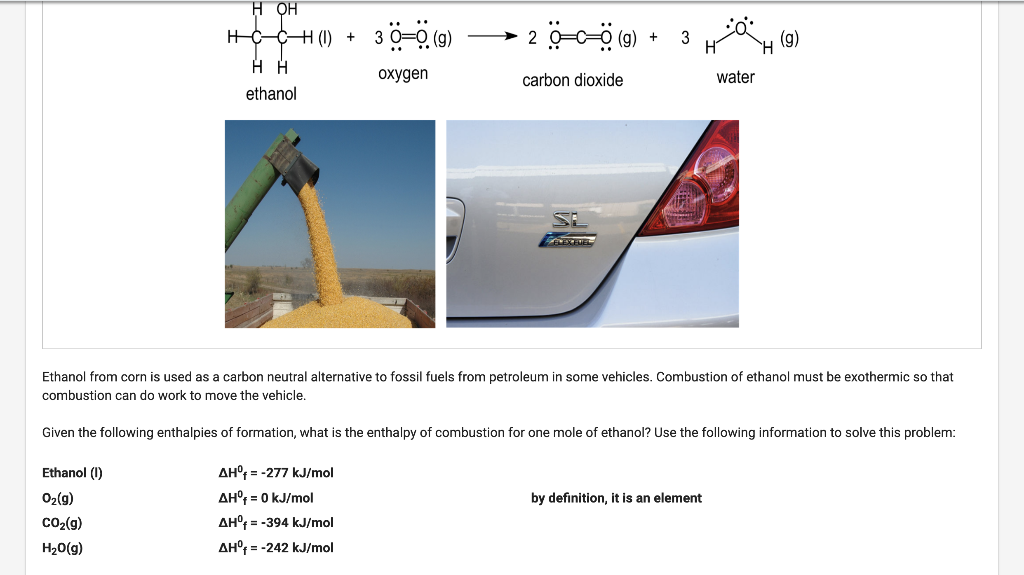
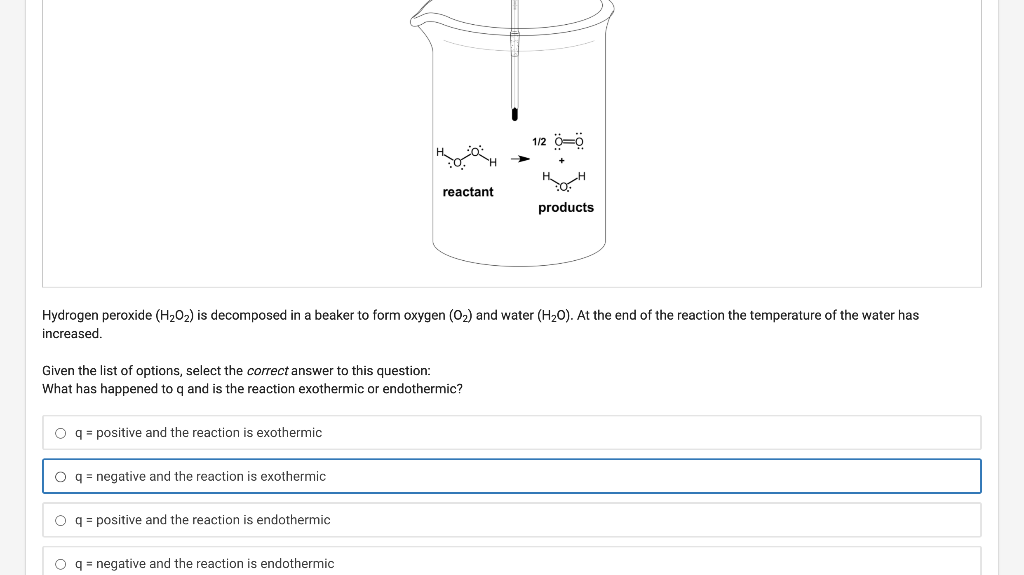
H OH H=CHCH (1) + 30=0 (9) ) 3 + (9) 2 (9) H HH oxygen carbon dioxide water ethanol si Ethanol from corn is used as a carbon neutral alternative to fossil fuels from petroleum in some vehicles. Combustion of ethanol must be exothermic so that combustion can do work to move the vehicle. Given the following enthalpies of formation, what is the enthalpy of combustion for one mole of ethanol? Use the following information to solve this problem: Ethanol (1) by definition, it is an element O2(g) CO2(g) H2O(g) Ahr = -277 kJ/mol AH = 0 kJ/mol AH = -394 kJ/mol AH = -242 kJ/mol Ethanol from corn is used as a carbon neutral alternative to fossil fuels from petroleum in some vehicles. Combustion of ethanol must be exothermic so that combustion can do work to move the vehicle. Given the following enthalpies of formation, what is the enthalpy of combustion for one mole of ethanol? Use the following information to solve this problem: Ethanol (1) by definition, it is an element O2(g) CO2(g) H2O(g) AH = -277 kJ/mol AHF = 0 kJ/mol AH = -394 kJ/mol AH = -242 kJ/mol O-359 kJ/mol 0 -659 kJ/mol 0 -1237 kJ/mol 0 -1791 kJ/mol Submit You have used 0 of 1 attempt 1/2 =0 PH reactant products Hydrogen peroxide (H2O2) is decomposed in a beaker to form oxygen (O2) and water (H20). At the end of the reaction the temperature of the water has increased. Given the list of options, select the correct answer to this question: What has happened to q and is the reaction exothermic or endothermic? O q = positive and the reaction is exothermic O q = negative and the reaction is exothermic O q = positive and the reaction is endothermic O q = negative and the reaction is endothermic
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


