Question: hello! how do i solve this question? please help Calculate the amount of energy in kilojoules needed to change 135g of water ice at 10C
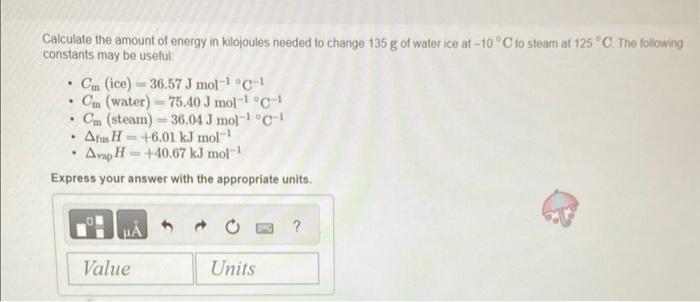
Calculate the amount of energy in kilojoules needed to change 135g of water ice at 10C to steam at 125C. The following constants may be useful: - Cm (ice) =36.57Jmol1C1 - Cm (water) =75.40Jmol1C1 - Cm( steam )=36.04Jmol1C1 - fusH=+6.01kJmol1 Express your answer with the appropriate units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


