Question: hello i need some help. Line AB-Ice warming or ice cooling; cal =g(TfolTiali)CSHice(+ for warm, for cool) Line BC-Ice to liquid water or liquid water
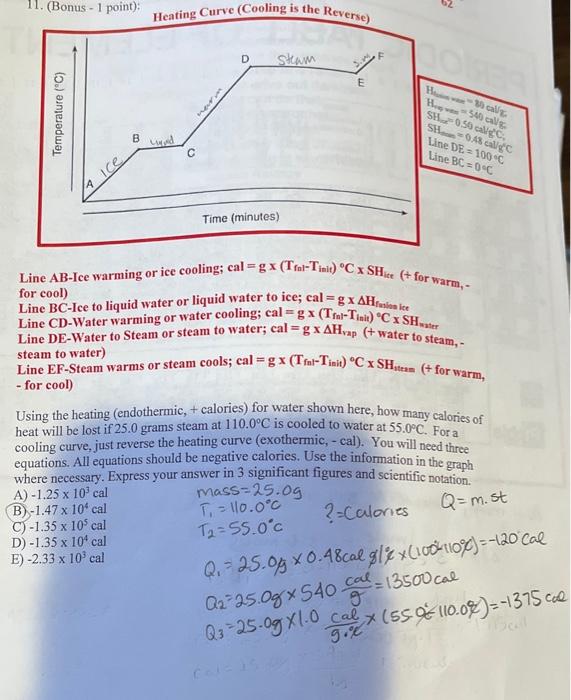
Line AB-Ice warming or ice cooling; cal =g(TfolTiali)CSHice(+ for warm, for cool) Line BC-Ice to liquid water or liquid water to ice; cal =gHfosionke Line CD-Water warming or water cooling; cal =g(TfalTinit)CSHwater Line DE-Water to Steam or steam to water; cal =gHvap ( + water to steam, steam to water) Line EF-Steam warms or steam cools; cal =g(TfalTinit)CSHttem(+ for warm, - for cool) Using the heating (endothermic, + calories) for water shown here, how many calories of heat will be lost if 25.0 grams steam at 110.0C is cooled to water at 55.0C. For a cooling curve, just reverse the heating curve (exothermic, - cal). You will need three equations. All equations should be negative calories. Use the information in the graph where necessary. Express your answer in 3 significant figures and scientific notation. A) 1.25103cal B) 1.47104cal C) 1.35105cal D) 1.35104cal E) 2.33103cal mass=25.0gT1=110.0CT2=55.0C?= Calories Q=m. st Q1=25.0g0.48calg/%(100110c)=120Cal Q2=25.0g540gcal=13500cal Q3=25.0g1.09.Ccal(55%=110.0%)=1375c
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


