Question: Help I'm stuck I've tried everything and keep getting it wrong The specific gravity of water at 20C is about 0.9982; the specific gravity of
Help I'm stuck I've tried everything and keep getting it wrong 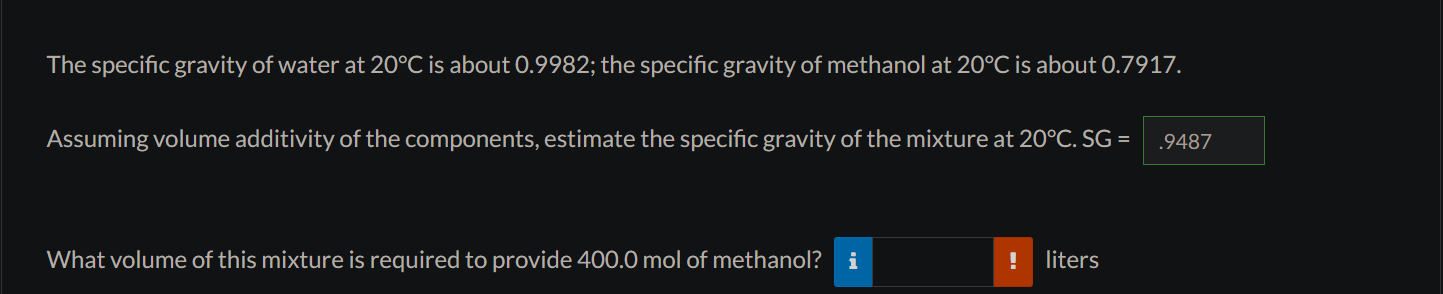
The specific gravity of water at 20C is about 0.9982; the specific gravity of methanol at 20C is about 0.7917. Assuming volume additivity of the components, estimate the specific gravity of the mixture at 20C.SG= What volume of this mixture is required to provide 400.0 mol of methanol? liters The specific gravity of water at 20C is about 0.9982; the specific gravity of methanol at 20C is about 0.7917. Assuming volume additivity of the components, estimate the specific gravity of the mixture at 20C.SG= What volume of this mixture is required to provide 400.0 mol of methanol? liters
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


