Question: help me fill out the bottom part Table 1: Time and Temperature for melting of Naphthalene and the mixture Unknown letter Mass of unknown compound
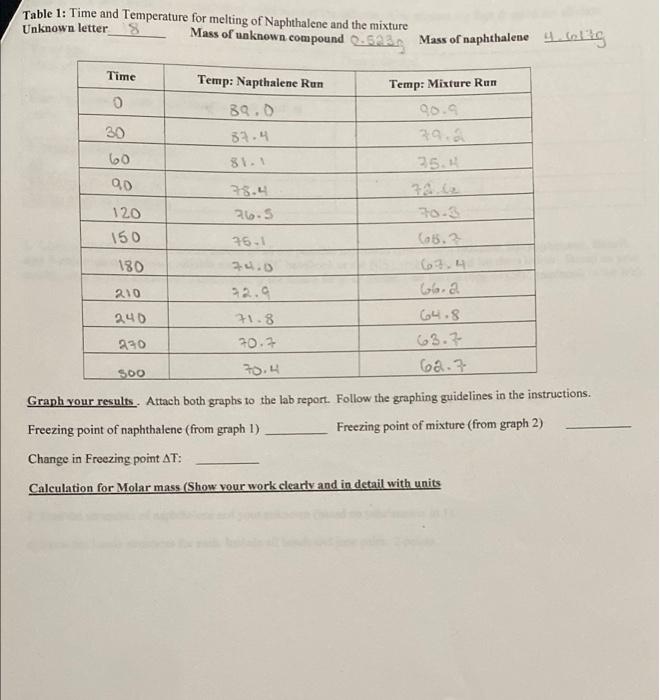
Table 1: Time and Temperature for melting of Naphthalene and the mixture Unknown letter Mass of unknown compound Gas Mass of naphthalene 4 inlig Time Temp: Napthalene Run 0 Temp: Mixture Run 90.9 39.0 52.4 30 811 25.4 60 90 28.4 26.5 120 150 25-1 180 34.0 92.9 210 10.3 (5.2 6.4 Gaa 64.8 63.7 60.7 240 71.8 70.7 230 SOO 70.4 Graph your results. Attach both graphs to the lab report. Follow the graphing guidelines in the instructions. Freezing point of naphthalene (from graph 1) Freezing point of mixture (from graph 2) Change in Freezing point AT: Calculation for Molar mass (Show your work clearly and in detail with units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


