Question: help me please L3 - Fractional Crystallization Introduction and Procedure One method for separating the components of a simple mixture is by filtration, taking advantage

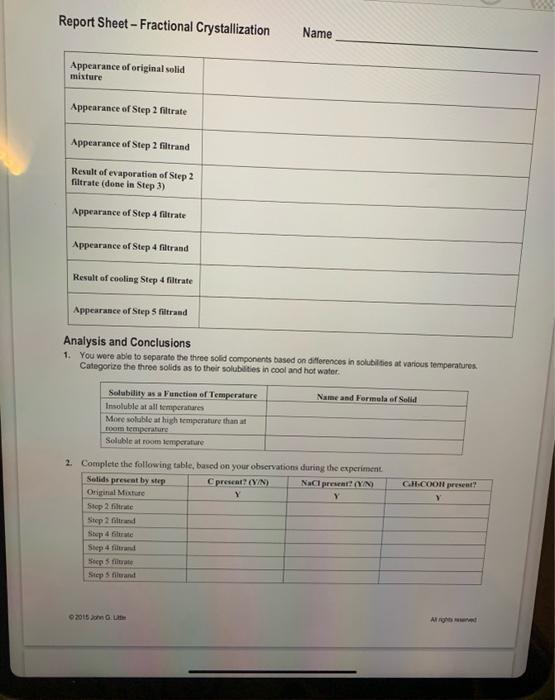

L3 - Fractional Crystallization Introduction and Procedure One method for separating the components of a simple mixture is by filtration, taking advantage of the difference in solubilities of solid substances. In this experiment, we start with a mixture of three solids. The first is benzoic acid ( C1H3COOH), weakly acidic, organic compound. The second is elemental carbon (C) in the form of powdered charcoal; carbon is a non-metallic element with a complex molecular structure. The last component is sodium chloride (NaCl), ordinary table salt. Both salt and benzoic acid are white solids, although one has a more distinguishable crystalline structure. Charcoal is a very finely divided solid. We will need to filter the mixture three diffetent times, so we start by labeling three paper filters as Step 2, Step 4, and Step 5. [Note: A pencil works much better than a pen for this.] 1. About 2.0g of the gray mixture is placed in a 100mL. beaker and about 30mL of water is added. What can you see about the solubility of the components of this mixture in water? The correct way to fold a paper filter is shown below. Essentially, it is folded in fourths, creating a sort of round-bottomed triangle. A filtration apparatus (ring stand, iron ring, wire triangle, funnel, "Step 2" paper filter) has been set up, Open the filter paper into a cone so that 3 layers are on one side, and one is on the other and place it in the funnel; you may find that wetting the filter with a few drops of distilled water makes it stay in place better. Place a second 100 -mL beaker beneath the funnel, with the lowest part of the funnel stem just touching the side of the receiver. This helps increase the rate of flow of filtrate through the filter. See Figures 1 and 2 for reminders on proper techique for folding filter papers and for filtering sugections. Figure 1: Preparing a Paper Filter 2. After stiring the mixture for a minute or two, we then filter as shown in Figure 2 , below. Note the use of a stirning rod to dirett the suream of liquid coming from the beaker spout. The liquid passing throagh the filler is called the filrrate, the insoluble residue remaining in the filter is called the filtrand. Deseribe the appearance of each in the appropriate blank of the Report Shect. Figure 2 Use a stirring rod to guide the stream of liquid. 3. A small amount of the filtrate from Step 2 is placed in an evaporating dish. The dish is placed on a wire sereen, supported by an iron ring and heat gently, uncovered, with a low bumer flame, until all or most of the water has evaporated. Observe the residue remaining in the dish does it appear to be all one type of erysal, or do you see more than one? 4. The gray-black, solid residue ("filtrand") from the Step 2 filter is transferred to a clean 150- or 250-mL, beaker. Use a distilled water wash boxtle to help with the transfer. Add distilled water as necesary to give yoa about 75 - 100mL of a liquid-solid suspension in the beaker. The beaker and contents are heated to a gentle boil which is maintained for about 2 minutes. To minimize cvaporation, we cover the beaker with a wath glass. While the heating is taking place, we set up a filtration apparatus using the "Step 4 " filier paper. A small beaker or Erlentneyer flask makes a suitable receiver for the filtrate. Using beaker tongs to protect the hands, we filter the hot mixture. When filtration is complete, the filtrate is allowed to cool for a few minutes, then we will put the receiver containing the filtrate in an ice bath. The "Step 4 " filter is set aside for later examination. The funnel is rinsed and the 3 te filter, "Step 5 ," is put in place. We examine the contents of the matecial in the Step 4 filter to determine whether it appears to be a single substance or a mixture. 5. As the previous filtrate cools, new white crystals will be fomed, although it may be necensary to use your stiming rod to scratch the walls of the container and indice crycallization. Once erystallization gets stanted, it goes neasonably fast, and should take only 5 10 minates. When it appears that erystallization is complete, and with the beaker and contents at or near 0C, we fitter oet the white crystals, using the "Step 5" fitter. The Step 5 filter is carefully removed from the funnel. We open the filter and use a hand lees (if available) to examine theie erystals and the ones reserved from steps 2 and 4 , In particular, you're looking for similarities and differences anoag the threw solids (ia addition to eolor) that you have recovered is this part of the experiment. In particular, note any differences in the appearance of the crystals of the twe white solids. 6. We discand the filtrate from Siep 5. Place all three filters, with their solid residues, is she waste coetainer provided for this experienent. Report Sheet - Fractional Crystallization Name Analysis and Conclusions 1. You wore able to separato the theee sold componenss based on diferences in solubilites at various temperaturea. Categorize the three solids as to their solubilites in cool and hot water. 2. Complete the following table, baved on your ohrervations alurinie the exncrimati: Qats pron 0. utim Mirgati named a. Basod on observations in this experiment, does it appear that any of the charcoul dissohved daring the heating in Step 4 ? Explain, citing evidence for your answer. b. Again, wsing your observations, could you tell whether all or jeat part of the bensuic acid dissolved during heating in Step 4 ? Explain. c. Of the three solid filtrands that were isolated, which soold yea expect to be the purent, and why? That is, which would be least likely to contain amounts of two (or more) of the three original solids? d. Again, referring to the thiee solid filirandr which one er cees can you be certain contaisol more than one of the hiree mixture coaponents? la other wordi, which of the thee is mest likely to be a mivture of at least two of the three solids in the original mixture? 4. We discarded the filtrale from Step 5 bet what mightyos cypet so see if we were to cvaporale nome of it as was done with the filtrate from Siep 2 Give a tenkes for yon antwer. L3 - Fractional Crystallization Introduction and Procedure One method for separating the components of a simple mixture is by filtration, taking advantage of the difference in solubilities of solid substances. In this experiment, we start with a mixture of three solids. The first is benzoic acid ( C1H3COOH), weakly acidic, organic compound. The second is elemental carbon (C) in the form of powdered charcoal; carbon is a non-metallic element with a complex molecular structure. The last component is sodium chloride (NaCl), ordinary table salt. Both salt and benzoic acid are white solids, although one has a more distinguishable crystalline structure. Charcoal is a very finely divided solid. We will need to filter the mixture three diffetent times, so we start by labeling three paper filters as Step 2, Step 4, and Step 5. [Note: A pencil works much better than a pen for this.] 1. About 2.0g of the gray mixture is placed in a 100mL. beaker and about 30mL of water is added. What can you see about the solubility of the components of this mixture in water? The correct way to fold a paper filter is shown below. Essentially, it is folded in fourths, creating a sort of round-bottomed triangle. A filtration apparatus (ring stand, iron ring, wire triangle, funnel, "Step 2" paper filter) has been set up, Open the filter paper into a cone so that 3 layers are on one side, and one is on the other and place it in the funnel; you may find that wetting the filter with a few drops of distilled water makes it stay in place better. Place a second 100 -mL beaker beneath the funnel, with the lowest part of the funnel stem just touching the side of the receiver. This helps increase the rate of flow of filtrate through the filter. See Figures 1 and 2 for reminders on proper techique for folding filter papers and for filtering sugections. Figure 1: Preparing a Paper Filter 2. After stiring the mixture for a minute or two, we then filter as shown in Figure 2 , below. Note the use of a stirning rod to dirett the suream of liquid coming from the beaker spout. The liquid passing throagh the filler is called the filrrate, the insoluble residue remaining in the filter is called the filtrand. Deseribe the appearance of each in the appropriate blank of the Report Shect. Figure 2 Use a stirring rod to guide the stream of liquid. 3. A small amount of the filtrate from Step 2 is placed in an evaporating dish. The dish is placed on a wire sereen, supported by an iron ring and heat gently, uncovered, with a low bumer flame, until all or most of the water has evaporated. Observe the residue remaining in the dish does it appear to be all one type of erysal, or do you see more than one? 4. The gray-black, solid residue ("filtrand") from the Step 2 filter is transferred to a clean 150- or 250-mL, beaker. Use a distilled water wash boxtle to help with the transfer. Add distilled water as necesary to give yoa about 75 - 100mL of a liquid-solid suspension in the beaker. The beaker and contents are heated to a gentle boil which is maintained for about 2 minutes. To minimize cvaporation, we cover the beaker with a wath glass. While the heating is taking place, we set up a filtration apparatus using the "Step 4 " filier paper. A small beaker or Erlentneyer flask makes a suitable receiver for the filtrate. Using beaker tongs to protect the hands, we filter the hot mixture. When filtration is complete, the filtrate is allowed to cool for a few minutes, then we will put the receiver containing the filtrate in an ice bath. The "Step 4 " filter is set aside for later examination. The funnel is rinsed and the 3 te filter, "Step 5 ," is put in place. We examine the contents of the matecial in the Step 4 filter to determine whether it appears to be a single substance or a mixture. 5. As the previous filtrate cools, new white crystals will be fomed, although it may be necensary to use your stiming rod to scratch the walls of the container and indice crycallization. Once erystallization gets stanted, it goes neasonably fast, and should take only 5 10 minates. When it appears that erystallization is complete, and with the beaker and contents at or near 0C, we fitter oet the white crystals, using the "Step 5" fitter. The Step 5 filter is carefully removed from the funnel. We open the filter and use a hand lees (if available) to examine theie erystals and the ones reserved from steps 2 and 4 , In particular, you're looking for similarities and differences anoag the threw solids (ia addition to eolor) that you have recovered is this part of the experiment. In particular, note any differences in the appearance of the crystals of the twe white solids. 6. We discand the filtrate from Siep 5. Place all three filters, with their solid residues, is she waste coetainer provided for this experienent. Report Sheet - Fractional Crystallization Name Analysis and Conclusions 1. You wore able to separato the theee sold componenss based on diferences in solubilites at various temperaturea. Categorize the three solids as to their solubilites in cool and hot water. 2. Complete the following table, baved on your ohrervations alurinie the exncrimati: Qats pron 0. utim Mirgati named a. Basod on observations in this experiment, does it appear that any of the charcoul dissohved daring the heating in Step 4 ? Explain, citing evidence for your answer. b. Again, wsing your observations, could you tell whether all or jeat part of the bensuic acid dissolved during heating in Step 4 ? Explain. c. Of the three solid filtrands that were isolated, which soold yea expect to be the purent, and why? That is, which would be least likely to contain amounts of two (or more) of the three original solids? d. Again, referring to the thiee solid filirandr which one er cees can you be certain contaisol more than one of the hiree mixture coaponents? la other wordi, which of the thee is mest likely to be a mivture of at least two of the three solids in the original mixture? 4. We discarded the filtrale from Step 5 bet what mightyos cypet so see if we were to cvaporale nome of it as was done with the filtrate from Siep 2 Give a tenkes for yon antwer
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


