Question: help om 17 please 980) 80 Dhe re. Temperature (C) 17. Fig. D.3 shows a temperature-composition diagram 60 for a mixture of two liquids exhibiting
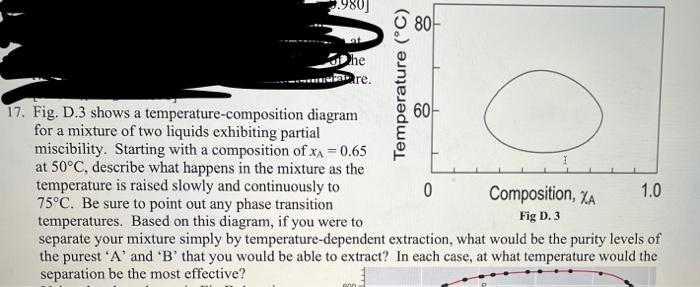
980) 80 Dhe re. Temperature (C) 17. Fig. D.3 shows a temperature-composition diagram 60 for a mixture of two liquids exhibiting partial miscibility. Starting with a composition of x^ = 0.65 at 50C, describe what happens in the mixture as the temperature is raised slowly and continuously to 0 Composition, XA 1.0 75C. Be sure to point out any phase transition temperatures. Based on this diagram, if you were to Fig D. 3 separate your mixture simply by temperature-dependent extraction, what would be the purity levels of the purest 'A' and 'B' that you would be able to extract? In each case, at what temperature would the separation be the most effective
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


