Question: Help please Calculate the freezing point and boiling point in each solution, assuming complete dissociation of the solute. Use Kf=1.86C/m and Kb=0.512C/m. Calculate the freezing
Help please 
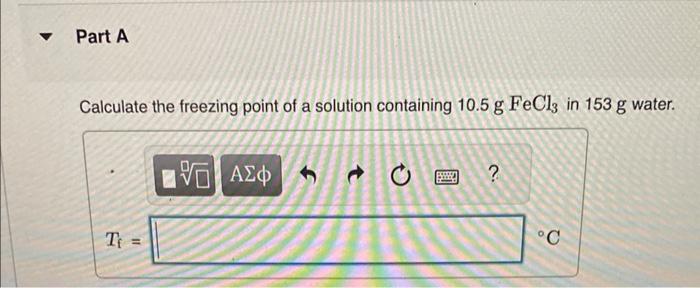
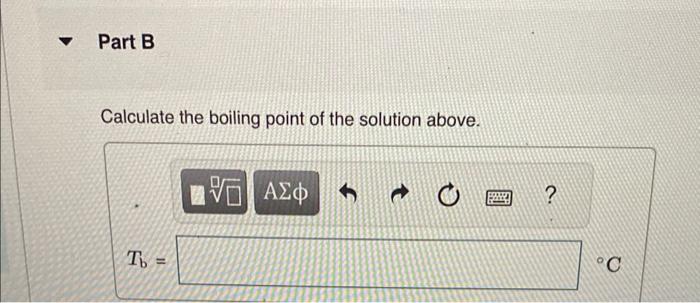
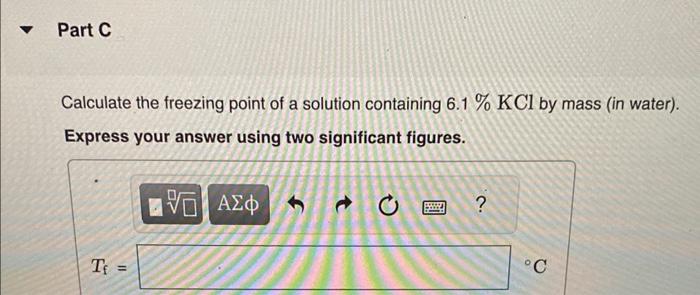
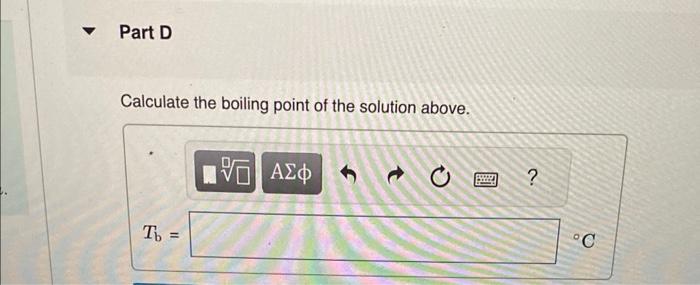
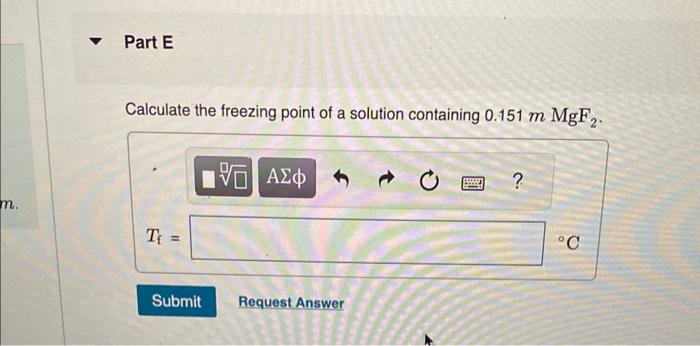
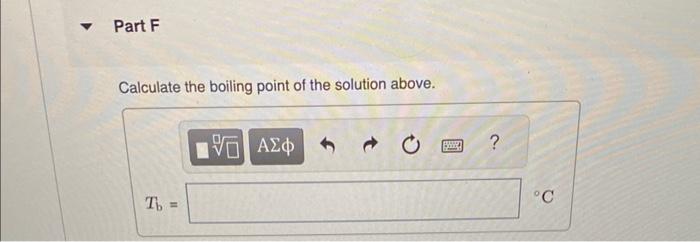







Calculate the freezing point and boiling point in each solution, assuming complete dissociation of the solute. Use Kf=1.86C/m and Kb=0.512C/m. Calculate the freezing point of a solution containing 10.5gFeCl3 in 153g water. Calculate the boiling point of the solution above. Calculate the freezing point of a solution containing 6.1%KCl by mass (in water). Express your answer using two significant figures. Calculate the boiling point of the solution above. Calculate the freezing point of a solution containing 0.151mMgF. Calculate the boiling point of the solution above
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


