Question: Help with the last part (section D) During lab, students mixed two solutions of soluble ions in a ceramic well to determine if a precipitate
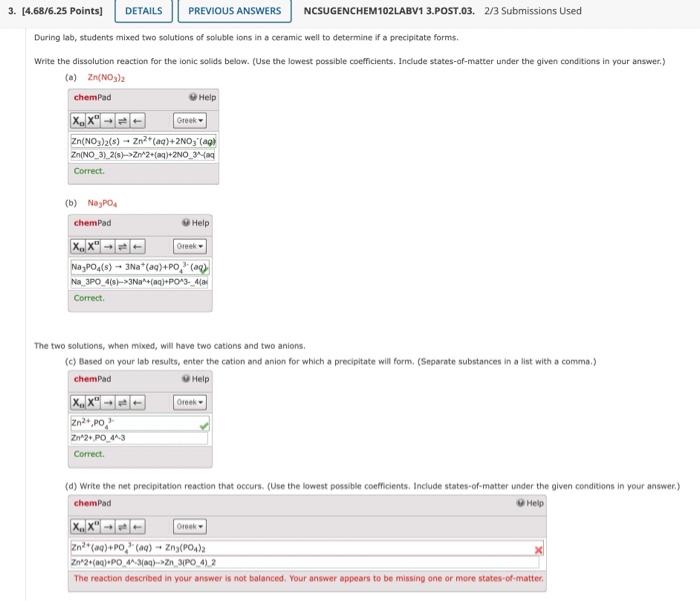
During lab, students mixed two solutions of soluble ions in a ceramic well to determine if a precipitate forms. Write the dissolution reaction for the ionic solids below. (Use the lowest possible coefficients. Include states-of-matter under the given conditions in your answer.) (a) 2n3(NO3)2 (b) Na3PO4 The two solutions, when mixed, will have two cations and two anions, (c) Based on your lab results, enter the cation and anion for which a precipitate will form. (Separate substances in a list with a comma.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


