Question: Here is how to do the problem: A dilute aqueous solution of calcium hydroxide at 25 C reacts with gaseous hydrogen chloride via the given
Here is how to do the problem:
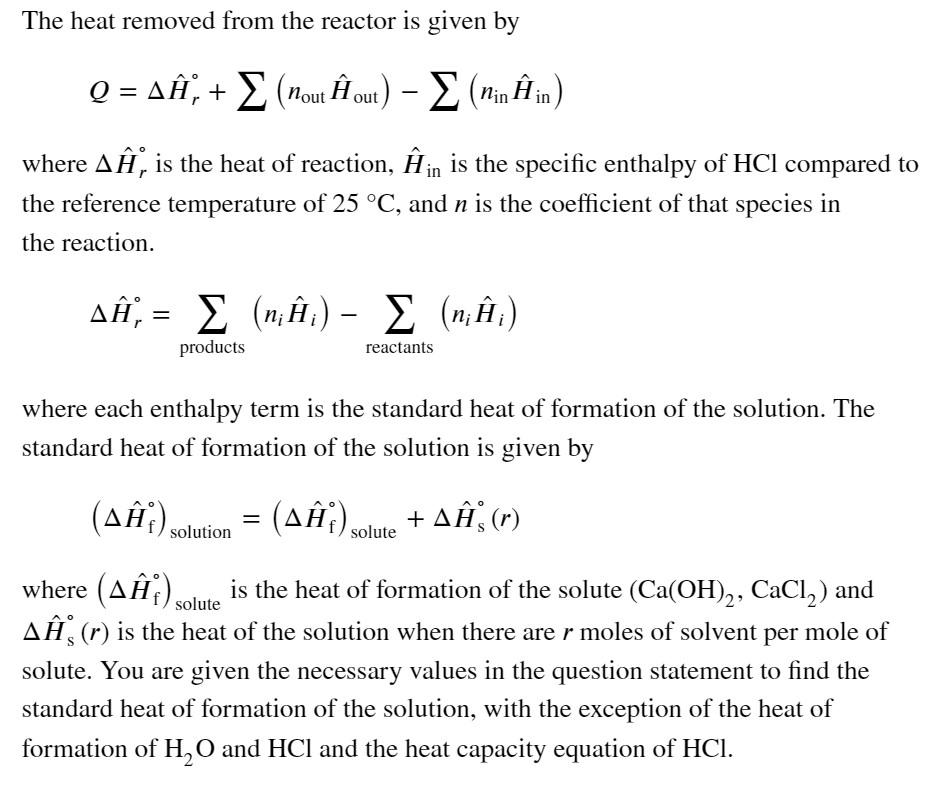
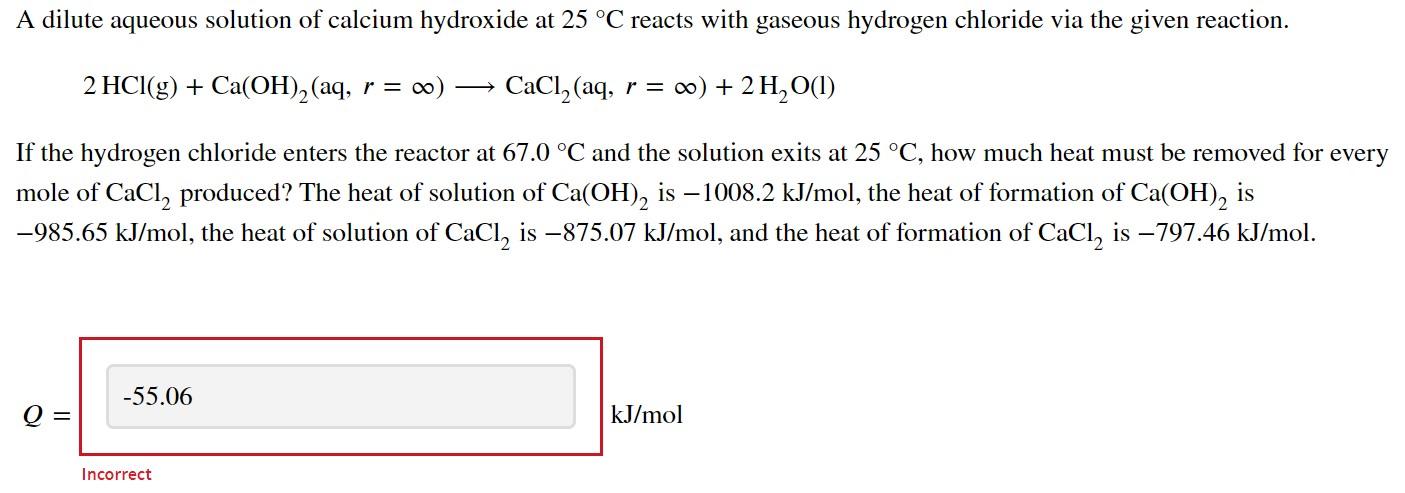
A dilute aqueous solution of calcium hydroxide at 25 C reacts with gaseous hydrogen chloride via the given reaction. 2 HCl(g) + Ca(OH)2 (aq, r = 0) CaCl, (aq, r = 0) + 2 H2O(1) If the hydrogen chloride enters the reactor at 67.0 C and the solution exits at 25 C, how much heat must be removed for every mole of CaCl, produced? The heat of solution of Ca(OH), is 1008.2 kJ/mol, the heat of formation of Ca(OH)2 is -985.65 kJ/mol, the heat of solution of CaCl, is 875.07 kJ/mol, and the heat of formation of CaCl, is 797.46 kJ/mol. -55.06 Q kJ/mol Incorrect The heat removed from the reactor is given by Q = A, + (nout out) - E (Minh in) where A, is the heat of reaction, in is the specific enthalpy of HCl compared to the reference temperature of 25 C, and n is the coefficient of that species in the reaction. A, = 2 (n;;) - E (n;;) products reactants where each enthalpy term is the standard heat of formation of the solution. The standard heat of formation of the solution is given by (A) solution = (A ) solute + A(r) solute where (AY) is the heat of formation of the solute (Ca(OH)2, CaCl,) and AS (r) is the heat of the solution when there are r moles of solvent per mole of solute. You are given the necessary values in the question statement to find the standard heat of formation of the solution, with the exception of the heat of formation of H,O and HCl and the heat capacity equation of HCl
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


