Question: Using the standard reduction potentials here, calculate the standard potential for the following cell in V (make sure you don't round your answer): Culs)Cu2(aq)||Mn2*(aq),H(aq)|Mn02(s)Pt(s)
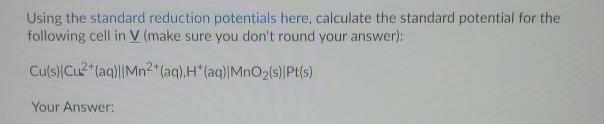
Using the standard reduction potentials here, calculate the standard potential for the following cell in V (make sure you don't round your answer): Culs)Cu2"(aq)||Mn2*(aq),H"(aq)|Mn02(s)Pt(s) Your Answer:
Step by Step Solution
3.50 Rating (160 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


