Question: How do we find the specific internal energy(h) in the steam tables according to this solution? A heat exchanger, shown in the figure, is used
How do we find the specific internal energy(h) in the steam tables according to this solution?
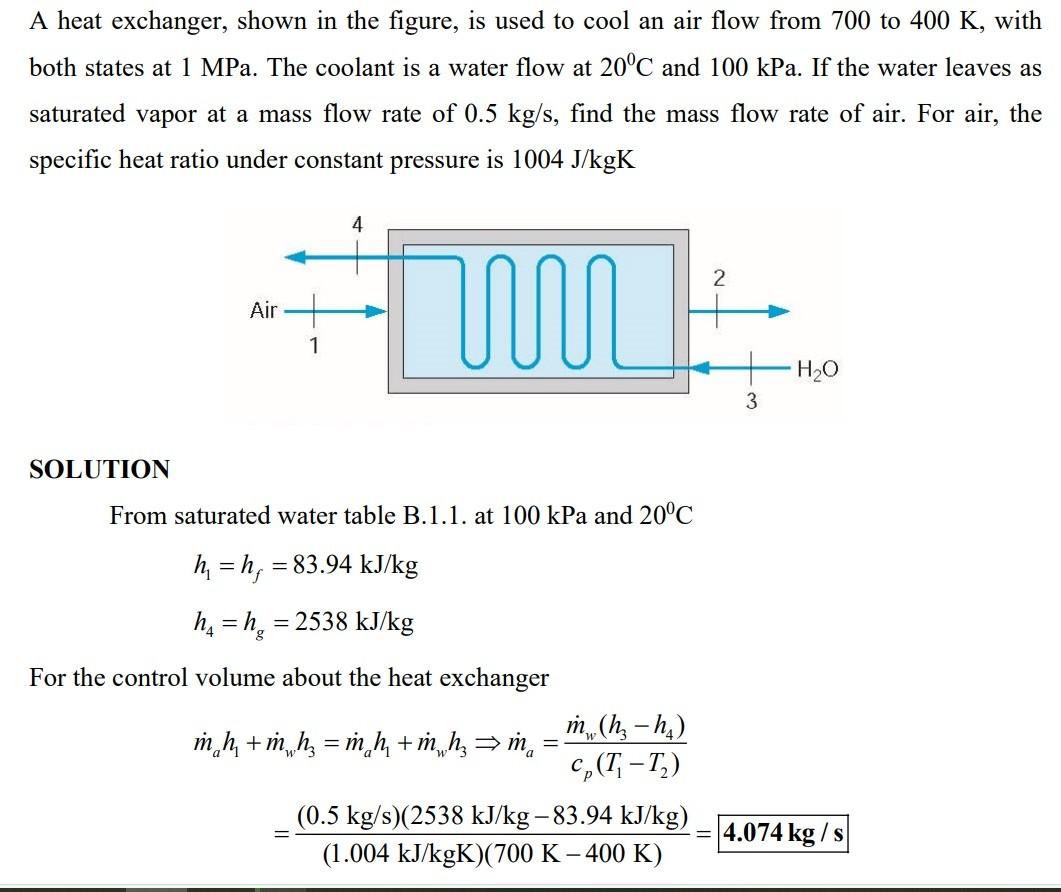
A heat exchanger, shown in the figure, is used to cool an air flow from 700 to 400K, with both states at 1MPa. The coolant is a water flow at 20C and 100kPa. If the water leaves as saturated vapor at a mass flow rate of 0.5kg/s, find the mass flow rate of air. For air, the specific heat ratio under constant pressure is 1004J/kgK SOLUTION From saturated water table B.1.1. at 100kPa and 20C h1=hf=83.94kJ/kgh4=hg=2538kJ/kg For the control volume about the heat exchanger mah1+mwh3=mah1+mwh3ma=cp(T1T2)mw(h3h4)=(1.004kJ/kgK)(700K400K)(0.5kg/s)(2538kJ/kg83.94kJ/kg)=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


