Question: A membrane with a thickness of 0.25 m is used to separate carbamazepine from aqueous solution. The concentration of carbamazepine on one side of
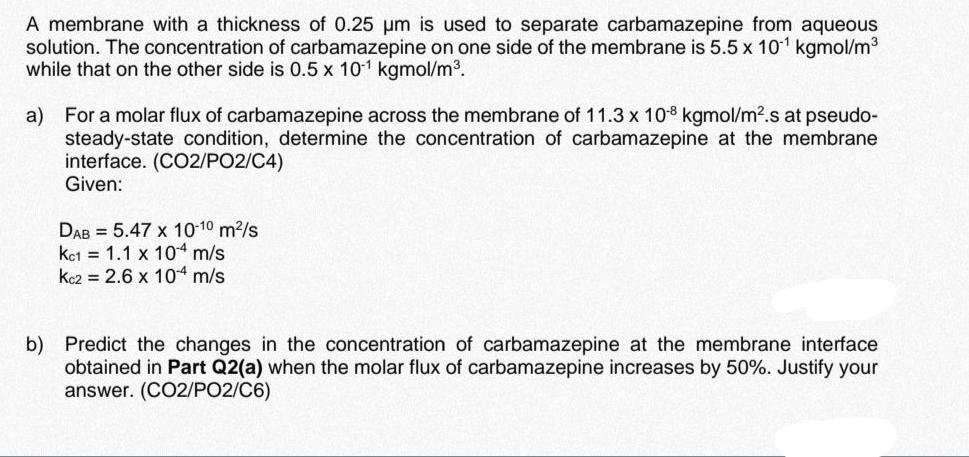
A membrane with a thickness of 0.25 m is used to separate carbamazepine from aqueous solution. The concentration of carbamazepine on one side of the membrane is 5.5 x 10 kgmol/m while that on the other side is 0.5 x 10 kgmol/m. a) For a molar flux of carbamazepine across the membrane of 11.3 x 108 kgmol/m.s at pseudo- steady-state condition, determine the concentration of carbamazepine at the membrane interface. (CO2/PO2/C4) Given: DAB 5.47 x 10-10 m/s Kc1 1.1 x 10-4 m/s Kc2= 2.6 x 10 m/s. b) Predict the changes in the concentration of carbamazepine at the membrane interface obtained in Part Q2(a) when the molar flux of carbamazepine increases by 50%. Justify your answer. (CO2/PO2/C6)
Step by Step Solution
3.45 Rating (158 Votes )
There are 3 Steps involved in it
Ficks first law of diffusion which connects the molar flux J to the concentration gradient and the diffusion coefficient can be applied to solve this ... View full answer

Get step-by-step solutions from verified subject matter experts


